Abstract
Background. Clinical trials indicate that the benefit of adding concurrent chemotherapy to radiotherapy of locally advanced non-small cell lung cancer (NSCLC) for fit elderly is similar to the benefit for younger patients. However, since elderly patients are under-represented in most trials, the results might be due to selection bias, thus reports from a cohort of consecutively treated patients are warranted. The current single institution study reports on the influence of age on survival of locally advanced NSCLC patients treated with radiotherapy combined with or without concurrent chemotherapy.
Material and methods. Altogether, 478 patients completed radical radiotherapy in doses of 60–66 Gy/30–33 fractions from 1995 to June 2012; 137 of the patients had concurrent chemotherapy. The data was analyzed in age groups < 60, 60–69, and ≥ 70 years.
Results. In the analyses of overall and lung cancer specific survival the hazard ratio was related to the use of concurrent chemotherapy was 0.49 (95% CI 0.29; 0.82), 0.68 (95% CI 0.48; 0.98) and 1.01 (95% CI 0.67; 1.51) for the age groups < 60, 60–69, and ≥ 70, respectively.
Conclusion. Use of concurrent chemotherapy to radiotherapy of locally advanced NSCLC was associated with a survival benefit in patient younger than 70 years which was not the case for patients older than 70 years, indicating the need to be careful when selecting elderly patients for concurrent chemo-radiation.
Background
When treating locally advanced non-small cell lung cancer (LA-NSCLC) with radical radiotherapy, concomitant chemo-radiation therapy (CRT) is superior to single modality radiotherapy and to radiotherapy preceded by neoadjuvant chemotherapy [Citation1,Citation2]. The median survival after addition of concurrent chemotherapy is about 17 months and the five-year survival is 15% [Citation1]. This is superior to the results of the studies reported in the 1980s showing median survivals typically of about 12 months, and five-year survival of less than 5% [Citation3]. Although data from published trials indicate that fit elderly benefit the same from the addition of chemotherapy in the treatment of LA-NSCLC as younger patients [Citation1,Citation4–7], elderly patients are under-represented in clinical trials. The impression of efficiency of CRT may to some extent be biased by the selection of included patients. It is therefore of interest if the improved survival by CRT among elderly patients can be reproduced in a cohort of consecutively treated patients.
Patients with LA-NSCLC have been treated in a standardized way with three-dimensional (3D) conformal radiotherapy for curative intent at Odense University Hospital since 1995. The prescribed doses have ranged from 60 to 66 Gy in 30–33 fractions. Prior to September 1999 the patients were treated with radiotherapy alone, and thereafter the standard treatment was neoadjuvant chemotherapy followed by radiotherapy, except for a limited number of patients who were treated with concomitant CRT as part of a trial. These patients are also included in this study. Since April 2004, neoadjuvant chemotherapy followed by CRT has been the standard treatment for patients in performance status 0–1.
This study reports the influence of patient age on survival in LA-NSCLC patients treated with and without CRT at Odense University Hospital in the period from 1995 to 2012.
Material and methods
Patients and their characteristics
NSCLC patients treated with radical radiotherapy at Odense University Hospital have been prospectively registered since 1995. Data from this cohort of patients have previously been published [Citation8–11].The current study includes all 478 patients treated between 1 September 1995 and 30 June 2012 who fulfilled radical radiotherapy in doses of 60–66 Gy and had histologically or cytologically confirmed LA-NSCLC. The tumor and nodes were staged according to AJJC/UICC 5th edition [Citation12] until 2009, and thereafter according to the 7th edition [Citation13]. Patients with T4N0-1 staged IIIB after the 5th edition have been re-coded to IIIA in this study, but otherwise no recoding has been performed. Patients with recurrent disease after surgery were staged as recurrent disease. Patients with malignant pleural effusion were not included in the study.
The data on radiotherapy, demographics, data on disease stage, pathology, basic clinical condition such performance status was prospectively recorded, and additional clinical data were obtained from patient charts. Patient characteristics are given in . The survival status of all patients was checked 1 February 2014 equivalent to a minimum follow-up (defined as time from start of treatment to end date of study) of more than 18 months. No patients were lost to follow-up. The follow-up schedule was every third month in two years and hereafter every six months for another three years. Medical history, clinical examination, and chest x-ray were obtained at each follow-up visit. If recurrent disease was suspected either clinical or radiographic, a computed tomography (CT) scan was conducted. From 2011, a CT scan was performed in conjunction with each follow-up for all patients. If necessary and clinically possible, biopsies were obtained from suspect lesions.
Table I. Patients characteristics of 478 patients with NSCLC.
Radiotherapy and chemotherapy
The patients were treated with 60–66 Gy in 30–33 fractions in 6–6½ weeks. All treatments were 3D planned until 2006 according to ICRU with advanced treatment planning system (Cadplan® to October 2001, Pinnacle® thereafter). From 2006 intensity-modulated technique (IMRT) gradually became standard of care and since 2007 4D CT planning was introduced as daily practice. The disease at the primary site and involved lymph nodes [gross tumor volume (GTV)] were outlined by a diagnostic radiologist. The GTV of primary tumor and nodes were expanded by 5–15 mm to create a clinical target volume (CTV). No elective node irradiation was performed, and all beams were treated at each fraction.
The concurrent chemotherapy consisted of docetaxel (20 mg/m2/week), oral vinorelbine (50 mg/3 times a week), carboplatin (AUC 5) – vinorelbine (i.v. 30 mg/m2 or oral 60–80 mg/m2 day 1 + 8), or carboplatin (AUC5) – etoposide [120 mg/m2 iv. day 1–3 × 2)].
Statistical analyses
Time to overall survival was calculated from time of commencement of radiotherapy to time of event or censoring. All patients alive at end of study were censored. In overall survival analyses all deaths were regarded as events, but additional lung cancer specific survival analyses were performed with only deaths of cancer or complication to treatment regarded as events. Survival analyses were made by Kaplan-Meier plots and two-sided log-rank test. The patients were divided in three age groups for the analyses of the effect of CRT: < 60, 60–69, and ≥ 70 years of age. Confounding variables that could be the underlying cause for significant univariate CRT results were tested in multivariate Cox proportional hazard analyses including age group, CRT, and the interaction term CRT × age group plus the variables showing a statistical significance less than 0.1 in relation to overall survival in univariate analyses (). Lung function was dictomized to below or above 75% of normal value since that value was near the median in the study population. Year of radiotherapy was dictomized in year < 2006/≥ 2006 since that year the use of IMRT became the standard radiotherapy technique at our center. A backwards stepwise selection of covariates for the final model was performed by SPSS ver. 21 using an exclusion criteria of p > 0.1. For all analyses p-values < 0.05 was considered statistical significant.
Table II. Univariate log rank analysis of the variables impact on overall survival in the total population.
Results
The group of patients treated with CRT consisted of 137 patients, while 341 patients were treated without concurrent chemotherapy. The latter group included 203 patients who had neoadjuvant chemotherapy. The median follow up time was 45 months in the CRT group, and 120 months in the patients treated without concurrent chemotherapy.
The age independent median, three- and five-year overall survival was 17.2 months, 24%, and 14%, respectively in the patients treated without concurrent chemotherapy, while the similar numbers for patients treated with CRT was 22.2 months, 35%, and 29% [HR = 0.70 (95% CI 0.55; 0.88, p = 0.002)].
In a univariate analysis the patients in the age groups < 60 years and 60–69 years had significant improved overall survival treated with CRT compared to the groups in which the patients did not receive concurrent chemotherapy (). The hazard ratio (HR) was 0.49 (95% CI 0.29; 0.82) in patients < 60 years and 0.68 (95% CI 0.48; 0.97) in patients 60–69 years. In patients ≥ 70 years no beneficial effect on survival was observed by adding concurrent chemotherapy (). The HR was 1.01 (95% CI 0.67; 1.51).
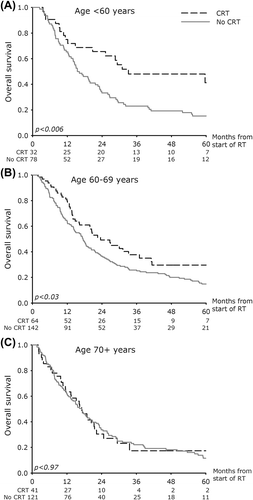
A backward elimination multivariate Cox proportional hazard model initially including CRT, age groups, CRT × Age, lung function, performance status, and year of treatment was performed. The Cox model resulted in the following significant variables: CRT, the CRT × Age group interaction term, lung function, year of treatment, and performance status, while age and gender were not statistically significant factors ().
Table III. Multivariate Cox proportional hazard regression in overall survival and lung cancer specific survival.
A trend to higher rate of deaths due to complications of the treatment was observed in CRT group of patients ≥ 70 years compared with rates in the other groups, but the number did not reach statistical significance (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.958529).
In univariate survival analyses using deaths of lung cancer or complication as event, the HR was 0.51 (95% 0.30; 0.87, p = 0.013) in patients < 60 years, 0.72 (95% 0.50; 1.03, p = 0.074) in patients 60–69 years, and 1.06 (95% 0.70; 1.62, p = 0.78) in patients ≥ 70 years. In the Cox analyses including the variables as in the analysis of overall survival, CRT, the CRT × Age group interaction term, performance status, and lung functions were significant factors, while age, gender, and years of treatment were statistically non-significant factors ().
Discussion
Several studies have demonstrated that in subgroups of elderly patients a reasonable long-term survival rate can be achieved with the use of advanced radiotherapy either alone or proceeded by neoadjuvant chemotherapy [Citation13–17]. Still, CRT is considered to be the standard treatment for patients with LA-NSCLC, as published guidelines do not make any exemptions for the elderly [Citation18].
Our results, however, indicate that not all elderly patients may benefit from CRT compared with radiotherapy without concurrent chemotherapy. This finding may seem in contrast to other studies on the use of CRT in an elderly population.
In a randomized study 197 patients older than 70 years received concurrent daily carboplatin together with radiotherapy 60 Gy/30 fractions or radiotherapy alone [Citation19]. Use of CRT was associated with an increased median survival from 16.9 to 22.4 months and HR was reported 0.68 (95% CI 0.47; 0.98).
In the meta-analysis by Aupérin et al. [Citation1], patients older than 70 seemed to benefit at least as much, if not more, from the use of CRT compared with younger patients.
Furthermore, a subanalysis of 104 patients of 70 years of age or older included in the randomized trial RTOG 94-10 was performed [Citation20]. These patients constituted 18% of all patients enrolled in the trial. A borderline significant improved survival was seen among patients receiving concurrent chemoradiation compared with sequential daily radiotherapy (p = 0.069). The median survival time was 22.4 months with concurrent daily radiotherapy, 16.4 months with concurrent twice daily radiotherapy, and 10.8 months for sequential daily radiotherapy. This was in contrast to previous trials conducted by the RTOG from 1988 to 1993, in which elderly patients did not benefit from increased therapeutic intensity.
Schild et al. published a retrospective combined analyses of two randomized trials performed by the North Central Cancer Treatment Group including 169 patients with stage III NSCLC 65 years old or older showing that patients receiving CRT had better overall survival than the patients treated with radiotherapy alone [Citation21].
Rocha Lima published a subanalysis of the influence of age a CALGB study of 191 patients randomized after neoadjuvant chemotherapy to concurrent carboplatin plus radiotherapy or radical radiotherapy alone [Citation14]. The 54 patients of 70 years or older had similar survival as the younger patients.
Other retrospective studies have demonstrated that addition of chemotherapy may lead to improved survival in the fit elderly patients. This was demonstrated in a retrospective study of 125 patients by Lee et al. [Citation6], but it is difficult to compare this study with ours since it did not distinguish between CRT and sequential use of chemotherapy and radiation. Besides, the radiation technique was less advanced compared with the techniques used in the present study.
Another retrospective study showing benefit of CRT in the elderly was published by Aridgides et al.. The study included 151 patients with stage III NSCLC treated with advanced radical radiotherapy with or without CRT including 61 patients of 70 years or older [Citation4]. Of these, 38 patients received CRT. Among the elderly patients, CRT was associated with superior survival compared with definitive radiotherapy alone (median survival 12.0 vs. 6.6 months) in univariate analyses. The authors concluded that appropriately selected elderly patients should receive definitive CRT. However, the study reported a very poor median survival after definitive radiotherapy without chemotherapy; in fact, no better than the survival after palliative radiotherapy of 6.2 months also reported in the paper, and this poor survival makes comparison to the present study difficult, as it hints to underlying differences in patient population and treatment technique.
The only study finding no effect of CRT among the elderly is a large Swedish multicenter retrospective study including NSCLC of all stages [Citation22]. In this study no benefit of CRT was found among 489 patients of 65 years of age or older treated with radical radiotherapy from 1990 to 2000.
Retrospective analyses should, however, always be interpreted with caution due to the risk of bias. That is also true for subanalysis of randomized trials like the analysis of RTOG94-10 and the trials by the North Central Cancer Treatment Group [Citation21], and the meta-analysis by Aupérin et al. [Citation22]. An obvious bias when interpreting the results is that the clinicians might be reluctant to offer concurrent chemotherapy to patients who appear fragile; thus these patients might not be included in trials. The studies therefore reflect only the fit elderly population, and therefore the elderly treated with CRT may have, for example better performance status than the patients treated without concurrent chemotherapy as seen in [Citation19] and [Citation21]. In the latter study less weight loss was also observed among patients receiving CRT. Thus, the proportion of elderly patients with LA-NSCLC included in the trials are smaller than proportion of all elderly patients having LA-NSCLC as seen in the meta-analyses by Aupérin et al. in which only 13% of the patients were 70 years or older [Citation1].
In retrospective analyses the group patients being treated with CRT is likely to have a better prognosis than the patients not offered concurrent chemotherapy, and it is expected that the survival should be better for the patients not offered concurrent chemotherapy. This is also the case in the present study. Part of the beneficial effect of CRT in patients less 70 year, is due to this bias, but it is less obvious why the same effect is not seen among patients ≥ 70 years. This observation may imply caution when treating the elderly with LA-NSCLC with CRT.
A problem treating the elderly may be the risk of complications. The current study demonstrated a trend to an increased risk of dying from complications to the treatment with high age both for patients treated with and without CRT. Several studies have reported increased risk of pneumonitis with age when concomitant CRT was used [Citation23–25]. This includes the RTOG 94-10 study [Citation20]. The QUANTEC paper on lung toxicity concluded that the risk was increased by age [Citation15]. A recently published meta-analysis of the risk of pneumonitis among 836 patients treated with CRT reported a trend of increased risk in patients older than 65 [Citation16]. An increased risk of toxicity after CRT was also observed in a trial of 243 patients with stage III NSCLC randomized to observation or consolidation docetaxel after fulfilled CRT to 59.4 Gy in 33 fractions [Citation5]. A significantly larger number of patients of 70 years or older discontinued CRT due to toxicity compared with younger patients (11% vs. 3%, p = 0.02). Although, this was not the case in CALGB subanalysis [Citation14], our study and other studies point at increased risk of toxicity to be a factor to consider when offering concurrent chemotherapy to the elderly patients.
The strength of the present study is that it is reporting on a large number of consecutively treated patients with LA-NSCLC treated with radical radiotherapy; including both the patients entered into a protocol, and the patients treated outside protocols. This study therefore reports on less rigorously selected patients compared with the reports from prospective phase II–III trials. The study is therefore reflecting results of every day practice in a single institution. Nevertheless, the result of this study is probably biased by selection bias of the patients, and to the evolution in the radiation technique during the period of almost 17 years that the patients were treatment in. It is important to emphasize that the findings in this study does not indicate that the fit elderly should not be treated with CRT, but individualized treatment should be based on detailed comprehensive geriatric assessment rather than on age itself. This report can therefore only be a reminder to be careful when identifying the elderly patients LA-NSCLC to CRT.
Conclusion
In this study CRT was associated with improved overall survival in patients < 70 years with LA-NSCLC. However, the effect of CRT was reduced for older patients, and for the patient group over 70 years no significant effect was observed, indicating the need to be careful when selecting elderly patients for concurrent chemo-radiation.
Supplementary material available online
Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.958529.
ionc_a_958529_sm9133.pdf
Download PDF (23.9 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work has been supported by CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research. The work is part of AgeCare (Academy of Geriatric Cancer Research) at Odense University Hospital.
References
- Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90.
- O’Rourke N, Roque IF, Farre BN, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2010;CD002140.
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lunge cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. Br Med J 1995;311:899–909.
- Aridgides PD, Janik A, Bogart JA, Duffy S, Rosenbaum P, Gajra A. Radiotherapy for stage III non-small-cell lung carcinoma in the elderly (age >/ = 70 years). Clin Lung Cancer 2013;14:674–9.
- Jalal SI, Riggs HD, Melnyk A, Richards D, Agarwala A, Neubauer M, et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: Analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol 2012;23:1730–8.
- Lee JH, Wu HG, Kim HJ, Kim DW, Lee SH, Kim TM, et al. Influence of comorbidities on the efficacy of radiotherapy with or without chemotherapy in elderly stage III non-small cell lung cancer patients. Cancer Res Treat 2012;44:242–50.
- Coate LE, Massey C, Hope A, Sacher A, Barrett K, Pierre A, et al. Treatment of the elderly when cure is the goal: The influence of age on treatment selection and efficacy for stage III non-small cell lung cancer. J Thorac Oncol 2011;6:537–44.
- Schytte T, Nielsen TB, Brink C, Hansen O. Pattern of local failure in non-small cell lung cancer patients treated with definitive radiotherapy. Acta Oncol 2014;53:336–41.
- Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: An updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552–8.
- Schytte T, Hansen O, Stolberg-Rohr T, Brink C. Cardiac toxicity and radiation dose to the heart in definitive treated non-small cell lung cancer. Acta Oncol 2010;49:1058–60.
- Nielsen M, Hansen O, Vach W. Attempts to predict the long-term decrease in lung function due to radiotherapy of non-small cell lung cancer. Radiother Oncol 2006;78:165–8.
- Sobin LH, Fleming ID. TNM classification of malignant tumors, 5th ed (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80:1803–4.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the tnm stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706–14.
- Rocha Lima CM, Herndon II JE, Kosty M, Clamon G, Green MR. Therapy choices among older patients with lung carcinoma: An evaluation of two trials of the cancer and leukemia group B. Cancer 2002;94:181–7.
- Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70–6.
- Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444–50.
- Wanders R, Steevens J, Botterweck A, Dingemans AM, Reymen B, Baardwijk A, et al. Treatment with curative intent of stage III non-small cell lung cancer patients of 75 years: A prospective population-based study. Eur J Cancer 2011;47:2691–7.
- Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143: e314S–40S.
- Atagi S, Kawahara M, Yokoyama A, Okamoto H, Yamamoto N, Ohe Y, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol 2012;13:671–8.
- Langer CJ, Hsu C, Curran WJ, Komaki R, Lee JS, Byhardt RB, et al. Elderly patients (pts) with locally advanced non-small cell lung cancer (LA-NSCLC) benefit from combined modality therapy: Secondary analysis of Radiation Therapy Oncology Group (RTOG) 94-10. Proc Ann Meet Am Soc Clin Oncol 2002;21:299a (Abstract 1193).
- Schild SE, Mandrekar SJ, Jatoi A, McGinnis WL, Stella PJ, Deming RL, et al. The value of combined-modality therapy in elderly patients with stage III nonsmall cell lung cancer. Cancer 2007;110:363–8.
- Holgersson G, Hoye E, Bergqvist M, Ekman S, Nyman J, Helsing M, et al. Swedish Lung Cancer Radiation Study Group: Predictive value of age at diagnosis for radiotherapy response in patients with non-small cell lung cancer. Acta Oncol 2012;51:759–67.
- Leprieur EG, Fernandez D, Chatellier G, Klotz S, Giraud P, Durdux C. Acute radiation pneumonitis after conformational radiotherapy for nonsmall cell lung cancer: Clinical, dosimetric, and associated-treatment risk factors. J Cancer Res Ther 2013;9:447–51.
- Salama JK, Stinchcombe TE, Gu L, Wang X, Morano K, Bogart JA, et al. Pulmonary toxicity in stage III non-small cell lung cancer patients treated with high-dose (74 Gy) 3-dimensional conformal thoracic radiotherapy and concurrent chemotherapy following induction chemotherapy: A secondary analysis of Cancer and Leukemia Group B (CALGB) trial 30105. Int J Radiat Oncol Biol Phys 2011;81:e269–74.
- Onishi H, Kuriyama K, Yamaguchi M, Komiyama T, Tanaka S, Araki T, et al. Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: A good local response but no good survival due to radiation pneumonitis. Lung Cancer 2003;40: 79–84.
