Abstract
Background. Trabectedin was in Europe approved for treatment of metastatic soft tissue sarcoma (STS) in 2007 based on results of a phase II study with relatively few patients. The purpose of this nationwide retrospective study was to assess efficacy and safety of using trabectedin in the entire unselected cohort of patients with metastatic sarcoma and to test known, as well as new prognostic factors that may affect overall survival (OS).
Patients and methods. Between January 2008 and April 2013, 117 patients were treated with trabectedin for metastatic sarcoma in the three specialized sarcoma centers in Denmark. Known prognostic factors such as age, gender and performance status, the histopathology as well as other new factors such as response to previous chemotherapy and hyponatremia were tested.
Results. Median age was 59 years. Lipo- and leiomysosarcomas (L-sarcomas) represented 43% of the cases and 18% had hyponatremia before the start of trabectedin. The median number of previous lines of chemotherapy was two (range 0–6) and the median number of chemotherapy cycles given before trabectedin was nine (range 0–85). The median number of trabectedin cycles was three (range 1–17). The median OS for the whole cohort was seven months. Poor performance status, non-L-sarcomas, and hyponatremia were statistically significant adverse prognostic factors with median survival of: 4, 5, and 2 months compared to 9, 12 and 13 months, respectively. Moreover, having achieved clinical benefit [complete response (CR), partial response (PR) or stable disease (SD)] from previous chemotherapy was a favorable prognostic factor for response to trabectedin. In multivariate analysis hyponatremia was the only independent significant poor prognostic factor affecting OS.
Conclusions. This retrospective study confirmed the previously published safety and efficacy of trabectedin in patients with metastatic sarcoma and showed hyponatremia to be a strong independent statistically significant poor prognostic factor.
Sarcomas are rare, heterogeneous, mesenchymal tumors accounting for approximately 1% of adult and 7% of childhood malignancies [Citation1,Citation2]. In Denmark, approximately 230–300 new cases of soft tissue sarcomas (STS) and bone sarcomas are diagnosed every year [Citation3]. Though the majority of patients with STS are diagnosed with localized disease approximately half the patients will develop local recurrence or distant metastasis in the course of the disease [Citation4].
Anthracycline and ifosfamide constitute the standard chemotherapy used for treating the majority of patients with metastatic STSs. As single agents, these standard drugs induce objective response rates in 15–30% of cases. Higher response rates are seen when these drugs are given in combination, although this does not impact survival [Citation5–7]. The toxic side effects associated with doxorubicin and/or ifosfamide may limit the use of these agents for effective palliation. Despite treatment with conventional chemotherapy, the prognosis for patients with metastatic sarcoma remains poor, with an estimated median survival of approximately one year from the start of first-line therapy [Citation8]. Trabectedin (ET743, Yondelis®) is a natural product of the Caribbean tunicate Ecteinascidia turbinata that is now produced semi- synthetically. This agent inhibits cell proliferation through binding to the minor groove of DNA and interacting with DNA transcription and tumor microenvironment [Citation9,Citation10].
Trabectedin was approved in the European Union in 2007 and later in other non-European countries for the treatment of patients with advanced STS after failure of therapy with an anthracycline and ifosfamide. The approval was based on efficacy data gathered from three single-arm phase II studies and one randomized phase II study comparing two different schedules of trabectedin. Later, further various retrospective publications have confirmed the efficacy of trabectedin in treatment of metastatic STS. Some of these trials showed particular efficacy of trabectedin in the so called L-sarcomas (Liposarcomas and Leiomyosarcomas) [Citation11–17].
Testing the efficacy of trabectedin in a large number of non-L-sarcoma patients and investigating other prognostic factors may be of value to clinical practice. To cast more light on the efficacy of trabectedin in the routine clinical setting, we conducted this national retrospective multicenter analysis, assessing the efficacy and tolerability of trabectedin in patients with different histological STS. We also included five patients with non-STS that had received treatment with trabectedin. We have also tested the impact of various prognostic factors expected to have an impact on treatment outcome. These factors include the ones well known at the time of planning this study (2011–2012) as well as p-sodium which has previously been shown to be negatively prognostic [Citation18,Citation19] in other cancer types including gastro-intestinal stromal tumor (GIST) [Citation20,Citation21].
The study was approved by the Data Protection Agency of Central Denmark region (J.nr. 1-16-02-101-12 and 2007-58-0010).
Patients and methods
The study cohort
This multi-center retrospective analysis was performed on all 117 consecutive patients (male n = 55; female n = 62) treated with trabectedin in three sarcoma centers in Denmark between January 2008 and April 2013. The great majority of patients (112) had histologically confirmed STS, one had osteosarcoma and four had chondrosarcoma. The data were obtained retrospectively by review of the patients’ medical journals and the national pathology database.
Dosage and administration
Trabectedin was administered at the standard dose of 1.5 mg/m² diluted in 500 ml NaCl as a continuous infusion over 24 hours using a central venous line. Solumedrol 100 mg was given prophylactically together with 5HT3 antagonists 30 minutes before trabectedin infusion. Pre- and post-hydration with NaCl 500 ml was given. G-CSF prophylaxis was applied according to clinical requirements and local practice. Cycles were repeated every three weeks.
Dose was reduced to 1.2 mg/m2 or 1.0 mg/m2 based on the investigators judgment. The current practice in Denmark is to reduce the dose if any grade 3–4 toxicity is reported including neutropenia with fever or infection, thrombocytopenia, or neurological toxicity. As for liver toxicity grade 2 elevation of alkaline phosphatase or bilirubin is usually an indication for dose reduction. In the elderly, it was possible to start the treatment with the reduced dose.
Assessments
Physical examination and standard laboratory parameters, including complete blood counts with differential counts, liver function, electrolyte and creatine kinase tests, were obtained according to local practice. In Denmark it is a standard practice to assess Performance Status (PS) using the ECOG/WHO criteria and report toxicities using the current Common Terminology Criteria for Adverse Events [Citation22]. Tumor response was assessed every three cycles with computed tomography and/or magnetic resonance tomography according to RECIST 1.0. If the patient received less than three series of trabectedin due to anything other than verified progressive disease (e.g. toxicity) the patient was classified as non-evaluable for response analysis.
Treatment was given until disease progression or discontinuation for other reasons, such as patient wish or unacceptable toxicity. In Western Denmark (Aarhus) a pause was sometimes allowed after 6–9 cycles with re-induction of treatment in case of progressive disease. In eastern Denmark (Herlev and Copenhagen) the treatment intervals could be extended to every four weeks in case of continuous clinical response after eight or nine cycles but with increasing toxicity.
Statistical analysis
Progression-free survival (PFS) was calculated from the date of trabectedin initiation until the date of progression or death. Overall survival (OS) was calculated from the start of therapy with trabectedin until death of any cause. Survival curves were plotted using the Kaplan-Meier method, and survival curves were compared by the log-rank test for the following factors; age (divided by median), gender, PS (0 & I vs. II), pathology (L-sarcomas vs. non-L-sarcomas), grade (I & II vs. III), metastatic site (pulmonary vs. extra pulmonary metastases), response to previous chemotherapy (attained clinical benefit vs. no clinical benefit) and plasma sodium (p-sodium; normal vs. hyponatremia). Clinical benefit was defined as stable disease (SD), partial response (PR) or complete response (CR) at evaluation of tumor response. The cut-off for p-sodium in this study was ≥ 137 mmol/l (reference interval 137–145 mmol/l equal to 137–145 mEq/l) tested on the day of start trabectedin or shortly before (≤ 7 days).
Multivariate analysis was carried out with Cox regression. All statistical analysis was done using SPSS-18.
Results
Patients and disease characteristics
Baseline characteristics are outlined in . Median age was 59 (range 19–87). Male to female ratio was 0.9. There was almost equal distribution of patients between Eastern (52%) and Western (48%) Denmark. Some poor prognostic factors were relatively prevalent such as poor PS in 17% of patients, concomitant cancer in 4% and low p-sodium in 18% of patients. Moreover, the favorable L-Sarcomas pathological subtypes represented only 43% of the patients and 89% had pathology grade 2 or 3. A median of 2 (range 0–6) chemotherapy lines were given before trabectedin with a median of nine cycles (range 0–85). Almost one third of the patients (31%) received not only anthracycline and ifosfamide but also at least one other chemotherapy before trabectedin.
Table I. Characteristics of the entire cohort of 117 patients.
Trabectedin treatment
Six patients (5%) received trabectedin as first-line treatment because anthracycline and/or ifosfamide were contraindicated or considered too toxic, whereas 60 (51%) and 19 (16%) patients received trabectedin as second and third chemotherapy line, respectively. The median number of trabectedin cycles administered was three (range 1–17).
Overall, 88 patients (79%) received at least two trabectedin cycles, ≥ 6 cycles were given to 29 (25%) patients, 15 (13%) of patients received trabectedin for at least nine cycles, and eight (7%) for 12 cycles or more.
Thirty-three patients (28%) experienced dose reduction. The most common cause of dose reduction was hematological toxicities (12 patients = 36%) followed by increased liver enzymes (10 patients = 30%).
Response and survival
Thirty-eight patients (33%) received less than three series of trabectedin and only 79 patients were therefore evaluable for response analysis. There were no patients with CR and only five patients (6%) with PR while 42 patients (52%) had stable disease. Among the evaluable patients with L-sarcomas (n = 40) 29 patients (73%) had clinical benefit. For evaluable patients with non-L-sarcomas (39 patients) there were 21 patients (54%) who obtained clinical benefit.
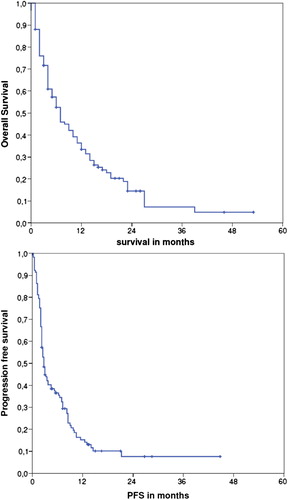
The median PFS and OS for all 117 patients were three and seven months, respectively, () while the median PFS and OS for the five patients with metastatic bone sarcomas were 2 and 10 months.
Figure 1. Overall and progression-free survival of the whole cohort of 117 patients with metastatic sarcoma treated with trabectedin as part of routine clinical practice in Denmark. Survival calculated from the date of starting trabectedin.
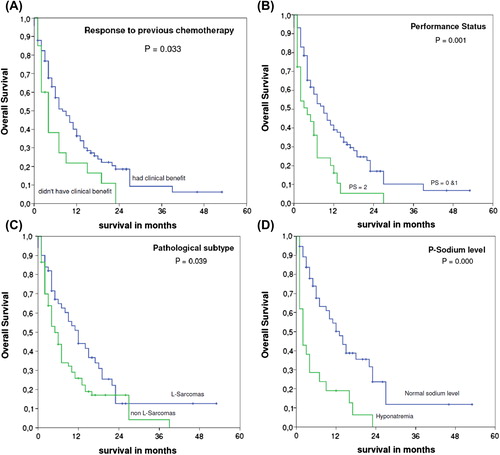
In a histological subgroup analysis, OS for patients with L-sarcomas (Liposarcomas and Leiomyosarcomas) was significantly (p = 0.039) higher than that in the other subgroups (12 and 5 months, respectively).
There were 20 patients who never had any clinical benefit of the pre-trabectedin chemotherapy. This particular group had significantly worse median OS of four months compared to eight months for patients who had clinical benefit to previous chemotherapy (p = 0.033).
Patients in PS 0-1 showed more than a double OS of nine months compared to four months for PS 2 (p = 0.001).
P-sodium measured at the day of start trabectedin or ≤ 7 days before was available for 77 patients. Patients with hyponatremia had a median OS of only two months which was significantly different (p < 0.0001) from a median OS of 13 months for patients with normal p-sodium.
Other prognostic factors: age, gender, pathology grade, and metastatic site were not found to be of any significant prognostic value to OS. Survival curves for the various significant factors are seen in .
Figure 2. Overall survival of the 117 patients with metastatic sarcoma treated with trabectedin according to various prognostic factors. (A) Response to previous chemotherapy, (B) PS, (C) Pathological subtype, (D) P-sodium level.
Multivariate analysis among all previously mentioned statistically significant factors showed hyponatremia to be the independent prognostic factor affecting OS ().
Table II. Multivariate analysis showing hyponatremia to be the only independent prognostic factor affecting OS among the tested variables.
Toxicity
A total of 35 patients (30%) didn't have any grade 3 or 4 toxicity of any kind. Among hematological toxicities, there was neutropenia in 19 (16%), neutropenia with fever in 10 (9%), anemia in seven (6%) and thrombocytopenia in five (4%). Twenty six patients (22%) had elevated liver enzymes and 46 (39%) experienced grade 3 fatigue. Treatment with trabectedin was discontinued due to excessive toxicity in 21 patients (18%). In this cohort we did not see any cases of rhabdomyolysis. Data for isolated elevation of creatine kinase was not collected.
There were three events of death associated with treatment of trabectedin (3%) all related to infections. One had pancytopenia, one had thrombocytopenia and the last patient had no grade 3 or 4 hematological toxicity. The deaths happened within three weeks of latest trabectedin dose. There was one extra death due to verified Clostridium perfringens sepsis occurring more than six weeks after trabectedin. The patient was not neutropenic and the episode was considered not related to trabectedin treatment.
Discussion
This study analyses the data of an unselected cohort representing all patients with verified STS treated with trabectedin in the period of January 2008 to April 2013 in Denmark and five patients with bone sarcomas treated with trabectedin in the same period. This group of patients were treated in the routine clinical setting outside of clinical trial. Patient characteristics therefore differed from the typical cohort in a randomized clinical trial in having a majority of patients with non-L-sarcomas, relatively high percent of patients in relatively poor PS, concomitant cancer, as well as electrolyte imbalance. These factors can explain the median OS seven months and the PFS three months from the start of trabectedin treatment, which is slightly lower than reported in other publications [Citation11,Citation12,Citation23,Citation24].
In this article response criteria are reported according to the RECIST criteria. In clinical practice however, Choi guidelines and the patients’ symptomatology are always taken into consideration for each individual patient before deciding whether to continue, pause or stop treatment. It is beyond the scope of this article to report on the extent by which Choi criteria (and not RECIST) were detrimental to treatment decision.
Tradition in western Denmark (Aarhus) has been leaning towards a preplanned maximum number of trabectedin cycles (6–9) with re-challenge in case of progression while patients in eastern Denmark (Copenhagen and Herlev) were treated until progression. There was no difference in outcome between patients treated in Western and Eastern Denmark and the survival curves are totally overlapping (data not shown). We cannot conclude, based on this material, that one strategy is better than the other. To harmonize with the international expert consensus, however, Aarhus has now changed its strategy towards the continuous approach.
Analysis of response rates showed efficacy of trabectedin in all pathological subtypes and confirmed literature data describing a superior response rate in L-sarcomas. We have included all patients treated with trabectedin in this analysis, which means that five non-STSs (one osteosarcoma, four chondrosarcomas) are represented in the data. The PFS and OS of these five patients were not different from the whole cohort and the small number of patients in this cohort prevented meaningful separate statistics of these five patients.
Interestingly, this study has shown that lack of response to previous chemotherapy is a poor prognostic in univariate analysis. Many new studies, in a variety of tumors, have shown that presence of cancer stem cells was a poor prognostic factor and a cause of persistent chemoresistance [Citation25–27]. It is possible therefore, that our results are related to the presence, in these resistant tumors, of an abundant cancer stem cell that exhibit a universal chemoresistance.
The study has also described another unique poor prognostic factor of clinical significance, namely hyponatremia. Though p-sodium data were only retrieved in 77 patients, the difference in OS between the two groups of normal versus low sodium levels was highly statistically significant in both uni- and multivariate analysis. Hyponatremia is known to be associated with shortened life expectancy both in malignant and non-malignant diseases [Citation18,Citation19,Citation21]. Moreover, it was previously reported to be a poor prognostic factor for patients with metastatic GIST [Citation20]. To our knowledge this is the first report showing its significance in patients with metastatic sarcoma. Hyponatremia has different known causes, i.e. drugs, alcohol, dyshydration, hyperglycemia, Syndrome of Inappropriate AntiDiuretic Hormone secretion (SIADH), inflammation, adrenal insufficiency and is often seen in geriatric patients but not all cases are explained. Hyponatremia could therefore be a sign of poor homeostasis and general weakness and it is unknown whether correction of asymptomatic hyponatremia could be of value or only treating the underlying cause has clinical meaning [Citation28]. It is important here to mention that other biomarkers such as pre-operative plasma fibrinogen, lymphocyte/monocyte ratio and C-reactive protein have recently been reported to be of importance to prognosis [Citation25,Citation29–32].
Conclusion
This retrospective study confirmed the previously published safety and efficacy of trabectedin in patients with metastatic sarcomas and showed that hyponatremia before trabectedin treatment is a strong independent statistically significant poor prognostic factor.
Acknowledgments
We acknowledge Henrik Roed, MD. D.Sc., Department of Oncology, The Finsen Center, Rigshospitalet, Copenhagen, Denmark, for letting us having access to the files of patients treated in the Finsen Center.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
- Olch AJ. Soft-tissue tumors. Pediatric radiotherapy – planning and treatment. London: CRC Press, Taylor & Francis Group; 2013. p 241.
- Statens Serum Institut. Cancerregistret 2012. Available from: http://www.ssi.dk/Sundhedsdataogit/Registre/Cancerregisteret.aspx. [Cited 26 February 2014].
- Weiss S, Goldblum J. Prognostic factors. Enzinger & Weiss's soft tissue tumors, 5th ed. Maryland Heights: Mosby Elsevier; 2008. p 18–31.
- Reichardt P. High-dose chemotherapy in adult soft tissue sarcoma. Crit Rev Oncol Hematol 2002;41:157–67.
- Sleijfer S, Ouali M, van Glabbeke M, Krarup-Hansen A, Rodenhuis S, Le Cesne A, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer 2010;46:72–83.
- Judson I, Verweij J, Gelderblom H, Hartmann JT, Schoffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol 2014;15:415–23.
- Verma S, Younus J, Stys-Norman D, Haynes AE, Blackstein M,Members of the Sarcoma Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 2008;34:339–47.
- D’Incalci M, Badri N, Galmarini CM, Allavena P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br J Cancer 2014;111: 646–50.
- Dossi R, Frapolli R, Di Giandomenico S, Paracchini L, Bozzi F, Brich S, et al. Antiangiogenic activity of trabectedin in myxoid liposarcoma: Involvement of host TIMP-1 and TIMP-2 and tumor thrombospondin-1. Int J Cancer Epub 2014 Jun 10.
- Demetri GD, Chawla SP, von Mehren M, Ritch P, Baker LH, Blay JY, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J Clin Oncol 2009;27:4188–96.
- Cesne AL, Judson I, Maki R, Grosso F, Schuetze S, Mehren MV, et al. Trabectedin is a feasible treatment for soft tissue sarcoma patients regardless of patient age: A retrospective pooled analysis of five phase II trials. Br J Cancer 2013; 109:1717–24.
- Garcia-Carbonero R, Supko JG, Manola J, Seiden MV, Harmon D, Ryan DP, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 2004;22:1480–90.
- Gronchi A, Bui BN, Bonvalot S, Pilotti S, Ferrari S, Hohenberger P, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 2012;23:771–6.
- Le Cesne A, Blay JY, Judson I, Van Oosterom A, Verweij J, Radford J, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 2005;23:576–84.
- Monk BJ, Blessing JA, Street DG, Muller CY, Burke JJ, Hensley ML. A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: A gynecologic oncology group study. Gynecol Oncol 2012;124:48–52.
- Yovine A, Riofrio M, Blay JY, Brain E, Alexandre J, Kahatt C, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol 2004; 22:890–9.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: Results from NHANES. Am J Med 2013;126:1127–37.e1.
- Schutz FA, Xie W, Donskov F, Sircar M, McDermott DF, Rini BI, et al. The impact of low serum sodium on treatment outcome of targeted therapy in metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Cancer Database Consortium. Eur Urol 2014; 65:723–30.
- Aggerholm-Pedersen N, Rasmussen P, Dybdahl H, Rossen P, Nielsen OS, Safwat A. Serum natrium determines outcome of treatment of advanced GIST with imatinib: A retrospective study of 80 patients from a single institution. ISRN Oncol 2011;2011:523915.
- Jeppesen AN, Jensen HK, Donskov F, Marcussen N, von der Maase H. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br J Cancer 2010;102:867–72.
- National Cancer Institute. NCI Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0. 2009; Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010- 06-14_QuickReference_5x7.pdf. [Cited 26 February 2014].
- Ploner F, Lamm W, Schur S, Eisterer W, Kuhr T, Lindorfer A, et al. The Austrian experience with trabectedin in non- selected patients with metastatic soft tissue sarcoma (STS). J Cancer Res Clin Oncol 2013;139:1337–42.
- Samuels BL, Chawla S, Patel S, von Mehren M, Hamm J, Kaiser PE, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: Results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
- Ahmed N, Abubaker K, Findlay J, Quinn M. Cancerous ovarian stem cells: Obscure targets for therapy but relevant to chemoresistance. J Cell Biochem 2013;114:21–34.
- Izumiya M, Kabashima A, Higuchi H, Igarashi T, Sakai G, Iizuka H, et al. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res 2012; 32:3847–53.
- Reers S, Pfannerstill AC, Maushagen R, Pries R, Wollenberg B. Stem cell profiling in head and neck cancer reveals an Oct-4 expressing subpopulation with properties of chemoresistance. Oral Oncol 2014;50:155–62.
- Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium: Do patients die from or with hyponatremia? Clin J Am Soc Nephrol 2011;6:960–5.
- Szkandera J, Pichler M, Liegl-Atzwanger B, Absenger G, Stotz M, Ploner F, et al. The elevated pre-operative plasma fibrinogen level is an independent negative prognostic factor for cancer-specific, disease-free and overall survival in soft-tissue sarcoma patients. J Surg Oncol Epub 2013 Oct 7.
- Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Samonigg H, et al. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer 2013;109:2316–22.
- Szkandera J, Pichler M, Absenger G, Stotz M, Arminger F, Weissmueller M, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg 2014;208:210–4.
- Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Friesenbichler J, et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer 2014;135:362–70.
