Abstract
Purpose. To determine the additional value of bone marrow biopsy (BMB) in the standard staging work-up of patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL), in terms of risk assessment and treatment planning.
Material and methods. A total of 113 consecutive patients with newly diagnosed DLBCL who had undergone standard pretreatment evaluation, including serum lactate dehydrogenase measurement, Eastern Cooperative Oncology Group performance status assessment, computed tomography or 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography, and BMB, were retrospectively included. National Comprehensive Cancer Network International Prognostic Index (NCCN-IPI) score and treatment strategy were determined in each patient, once without and once with taking into account BMB results. Numbers and percentages of BMB-induced changes on NCCN-IPI-based risk stratification (i.e. formation of low, low-intermediate, high-intermediate, and high risk groups) and choice of treatment were calculated, along with 95% confidence intervals (CIs).
Results. BMB was positive in 18 of 113 patients (15.9%, 95% CI 10.2–23.9 %). BMB-induced changes on NCCI-IPI-based risk stratification occurred in 9 of 113 patients (8.0%, 95% CI 4.1–14.6%). Five patients were upstaged from low-intermediate to high-intermediate risk, and four patients were upstaged from high-intermediate to high risk. BMB findings changed treatment planning in none of the 113 patients (0.0%, 95% CI 0.0–4.0%).
Conclusion. Although BMB results upstaged the NCCN-IPI-based risk stratification in a small number of cases, this did not have any therapeutic implications in our patient series. These findings support the omission of BMB from routine staging of newly diagnosed DLBCL in the current risk stratification and treatment era.
Diffuse large B-cell lymphoma (DLBCL) comprises approximately 30–35% of all non-Hodgkin lymphomas [Citation1,Citation2]. Present guidelines recommend acquiring a (blind) bone marrow biopsy (BMB) of the posterior iliac crest in all patients with newly diagnosed DLBCL who qualify for curative therapy [Citation3]. However, BMB is invasive and painful [Citation4], and has a non-negligible risk of adverse events (hemorrhage being the most common [Citation5]). In addition, bone marrow should be fixed and decalcified before it can be histologically examined [Citation6], which is a time- consuming procedure that may cause treatment delay. To justify performing a BMB in all patients with newly diagnosed DLBCL, the benefits associated with BMB should outweigh its disadvantages.
BMB serves several purposes in the evaluation of DLBCL in the pretreatment setting. First, BMB may aid in diagnosing DLBCL in patients under suspicion of lymphoma without appropriate extramedullary lesions for biopsy. Second, histologically proven lymphomatous bone marrow involvement, has been established as an adverse prognostic factor [Citation7,Citation8] and has been incorporated into the National Comprehensive Cancer Network (NCCN) International Prognostic Index (IPI) for risk stratification [Citation9]. Third, since determination of bone marrow involvement indicates stage IV disease, it may result in selecting a more intense therapeutic regimen. Overall, the incidence of (non-image guided) lymphoma-positive marrow histology in newly diagnosed DLBCL is low, approximately 13–17% [Citation7,Citation8,Citation10]. Importantly, BMB-induced therapeutic consequences are more likely to occur in patients with clinical and imaging-based early stage disease (particularly in Ann Arbor stage I disease) than in advanced stage disease. However, bone marrow involvement is less common in (presumed) early stage disease compared to advanced stage disease [Citation7,Citation10,Citation11]. Consequently, it can be hypothesized that the majority of BMBs do not have any clinical consequences for risk stratification and choice of treatment, and that these patients may be spared an invasive BMB.
The purpose of this study was therefore to determine the additional value of BMB in the standard staging work-up of patients with newly diagnosed DLBCL, in terms of risk assessment and treatment planning.
Material and methods
Study design and patient population
This retrospective study was approved by the local institutional review board and a waiver for written informed consent was granted. A search was performed in the database of the Meander Medical Center to identify all consecutive patients who were newly diagnosed with DLBCL between January 2004 and February 2014. Study inclusion criteria were: newly diagnosed and histologically proven DLBCL by means of (excisional) biopsy of an extramedullary site, availability of LDH measurement, availability of ECOG performance status score, availability of CT or FDG-PET/CT from skull base to upper thigh, and availability of (blind) BMB of the posterior iliac crest. Study exclusion criteria were: diagnosis of DLBCL solely based on BMB findings, primary mediastinal DLBCL (which is regarded as a separate disease entity), previously treated/relapsed lymphoma, transformed lymphoma (except primary DLBCL patients with discordant bone marrow histology), coexistence of another lymphoma subtype in the diagnostic biopsy, lack of serum LDH measurement, lack of ECOG performance status score, lack of CT or FDG-PET/CT from skull base to upper thigh, lack of BMB or poor (non-diagnostic) BMB quality, time interval between CT or FDG-PET/CT and BMB exceeding 30 days, and start of treatment before the above-mentioned standard pretreatment evaluation procedures.
BMB
Blind unilateral BMB of the right posterior iliac crest was performed in all patients by using a biopsy needle (Angiotech T-Lok, Medical Device Technologies) that is designed to ideally provide a 20-mm long 8-gauge (3.4-mm diameter) specimen. BMBs were acquired by different hematologists and evaluated by different hematopathologists according to the WHO criteria [Citation12], as part of routine clinical care. BMB was considered positive if either large-cell (concordant) or small-cell (discordant) lymphomatous involvement was present.
CT and FDG-PET/CT
CT scanning only was performed in all patients who were diagnosed with DLBCL before September 2007 (N = 29), using a 16-detector row CT system (Somatom Sensation 16, Siemens Healthcare). Patients ingested an oral contrast agent and were intravenously administered a non-ionic iodinated contrast agent before full-dose portal venous phase CT scanning was performed from skull base to upper thigh. FDG-PET/CT scanning was performed in all patients who were diagnosed with DLBCL after September 2007 (N = 84), using a 40-detector row PET/CT system (Biograph 40 TruePoint PET/CT, Siemens Healthcare). Following a fasting period of six hours, 3 MBq/kg body weight of FDG was injected, 60 minutes after which low-dose FDG-PET/CT scanning was performed from upper thigh to skull base. A subset of 60 patients also underwent full-dose oral and intravenous contrast-enhanced portal venous phase CT scanning from skull base to upper thigh, in addition to low-dose FDG-PET/CT, using the same PET/CT system.
An experienced reader (T.C.K.), who was blinded to clinical and laboratory findings, findings of other imaging modalities, histological findings, and patient outcome, reevaluated all pretreatment CT and FDG-PET/CT scans of included patients. Lymph nodes with a short-axis diameter exceeding 10 mm, and extranodal sites with abnormal attenuation, nodules or masses compatible with lymphomatous involvement, were considered positive at CT. Any FDG uptake exceeding background FDG uptake in a location incompatible with normal anatomic and physiologic conditions was considered positive for lymphomatous involvement at FDG-PET/CT.
Ann Arbor staging
Using clinical, CT or FDG-PET/CT, and available non-BMB histological findings, an Ann Arbor stage (I–IV) was assigned in each patient [Citation13]. BMB findings were ignored for Ann Arbor staging for the purpose of this study. Also note that imaging-based bone marrow involvement was also ignored for Ann Arbor staging, because the FDG-PET/CT based bone marrow involvement has been reported to have no prognostic implications [Citation14,Citation15].
NCCN-IP-based risk stratification
The NCCN-IPI uses a maximum of eight scoring points for categorized age > 40–60 (1 point), > 60–75 (2 points) and > 75 years (3 points), LDH ratio > 1 (1 point) and > 3 (2 points) times the upper limit of normal in addition to extranodal disease in major organs (either bone marrow, central nervous system, liver/gastrointestinal tract or lung) (1 point), Ann Arbor stage III/IV (1 point), and ECOG performance status ≥ 2 (1 point) [Citation9]. Note that imaging-based bone marrow involvement only was ignored for the purpose of Ann Arbor staging and determination of extranodal disease in major organs, because the NCCN-IPI requires bone marrow involvement to be histologically proven [Citation9]. Two NCCN-IPI scores were calculated in each patient, one time without and one time with taking into account BMB results. Subsequently, each patient was classified into one of the four following risk groups: low risk (scores 0–1), low-intermediate risk (scores 2–3), high-intermediate risk (scores 4–5), and high risk (scores 6–8), without and one time with taking into account BMB results.
Treatment consequences
All BMB-positive cases were reviewed by an experienced hematologist (R.F.), who had access to all pretreatment clinical, laboratory, imaging, and histological findings, but who was blinded to patient follow-up/outcome. The hematologist determined if and how the lymphoma-positive BMB findings changed treatment planning in these patients compared to the treatment strategy based on the already available clinical, laboratory, imaging, and available non-BMB histological findings.
Statistical analysis
Number and proportion of patients with a positive BMB among the entire study population were calculated, along with 95% confidence intervals (CIs). Number and proportion of positive BMBs among Ann Arbor stage I, II, III, and IV disease were calculated, along with 95% CIs. Numbers and percentages of BMB-induced changes in NCCN-IPI-based risk stratification (i.e. formation of low, low-intermediate, high-intermediate, and high risk groups) and choice of treatment were calculated, along with 95% CIs. Statistical analyses were executed using MedCalc statistical software version 12.6.0 (Ostend, Belgium).
Results
Patients
A total of 212 consecutive patients presented with newly diagnosed DLBCL at our institution between January 2004 and February 2014. Of these 212 patients, two were excluded because the diagnosis of DLBCL was solely based on BMB findings, four were excluded because of primary mediastinal DLBCL, 15 were excluded because of transformed lymphoma, 18 were excluded because of coexistence of another lymphoma subtype in the diagnostic/extramedullary biopsy, five were excluded because of another malignancy within the previous five years, 23 were excluded because of non- availability of CT or FDG-PET/CT from skull base to upper thigh, 23 were excluded because of lack of BMB, five were excluded because of poor, non-diagnostic BMB quality, and four were excluded because the time interval between CT and BMB exceeded 30 days. Finally, 113 patient (61 men and 52 women, mean age: 65.3 ± 14.1 years, age range: 24–88 years) were included. Of these 113 patients, 29 (25.7%) were diagnosed with DLBCL before September 2007 and had undergone CT only, whereas 84 (74.3%) were diagnosed with DLBCL after September 2007 and had undergone FDG-PET/CT. Further, detailed patient characteristics are presented in .
Table I. Characteristics of included patients.
Distribution of positive BMBs among different Ann Arbor stages
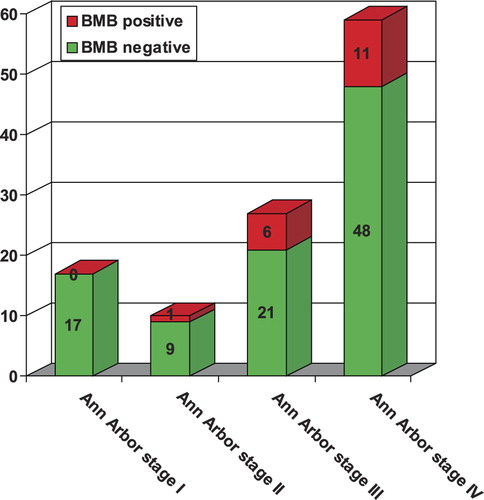
BMB was positive for lymphoma in 18 of 113 patients (15.9%, 95% CI 10.2–23.9%). Of these 18 BMB-positive cases, 12 had large cells, four had small cells, and two had an indeterminate cell type (i.e. discrimination between large and small cells was not possible) in the marrow specimen. None of the 17 patients with Ann Arbor stage I disease had a positive BMB (0.0%, 95% CI 0.0–21.6%), 1 of the 10 patients with Ann Arbor stage II disease had a positive BMB (10%, 95% CI 0.0–42.6%), six of the 27 patients with Ann Arbor stage III disease had a positive BMB (22.2%, 95% CI 10.3–41.1%), and 11 of the 59 patients with Ann Arbor stage IV disease had a positive BMB (18.6%, 95% CI 10.6–30.6%) ().
BMB-induced changes on NCCN-IPI-based risk stratification
BMB-induced changes on NCCI-IPI-based risk stratification occurred in nine of 113 patients (8.0%, 95% CI 4.1–14.6%). Five patients were upstaged from the low-intermediate to the high-intermediate risk group and four patients were upstaged from the high-intermediate to the high risk group ().
Table II. Cross-tabulation of NCCI-IPI-based risk stratification without versus with taking into account BMB results.
BMB-induced changes on treatment planning
BMB findings changed treatment planning in none of the 113 patients (0.0%, 95% CI 0.0–4.0%).
Discussion
The results of this study show that BMB is positive in approximately 15.9% of patients with newly diagnosed DLBCL, regardless of disease stage. BMB results upstaged the NCCN-IPI-based risk stratification in a small minority (8.0%) of patients, either from low-intermediate to high-intermediate risk, or from high-intermediate to high risk. Importantly, positive BMBs were present far more frequently in advanced stage disease than in early stage disease, with the majority already having Ann Arbor stage IV disease based on CT or FDG-PET/CT studies. None of the patients with Ann Arbor stage I disease had a positive BMB, therefore in none of these patients would treatment planning have been changed from short-course (three cycles) R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine and prednisone) chemoimmunotherapy with additional radiation therapy to prolonged (six or eight cycles) R-CHOP chemoimmunotherapy only. In addition, although a minority of patients was upstaged from Ann Arbor stage II or III disease to Ann Arbor stage IV disease based on BMB results, this did not have therapeutic consequences in any of these patients either.
One previous study by Lim et al. challenged the need to routinely perform BMB in all patients with newly diagnosed DLBCL [Citation11]. In that study which only included patients with CT-based early stage (Ann Arbor stages I and II) disease, seven of 192 patients (3.6%) had a positive BMB. It was reported that the parameters hemoglobine < 10 g/dl (p = 0.02), white blood cell count < 4 × 109/L (p = 0.007) and bulky disease (p = 0.06) were predictive of bone marrow involvement. Among the 120 patients without any of these three factors, only one patient had bone marrow involvement (0.8%, 95% CI 0.0–4.6%). Accordingly, the absence of all three factors provided a negative predictive value of 99.2%. Lim et al. concluded that BMB may be safely omitted in selected patients with early stage DLBCL. Unfortunately, it was not reported how many patients with radiological Ann Arbor stage I disease had a positive BMB, because BMB results in this group of patients may have important therapeutic implications (i.e. a positive BMB would result in a change from short-course R-CHOP chemoimmunotherapy with additional radiation therapy to prolonged R-CHOP chemoimmunotherapy only). In addition, BMB-induced changes on risk stratification were not assessed [Citation11]. Although the present study included less patients with early stage disease than the study by Lim et al. [Citation11], it provides important new information on the additional utility of BMB with regard to risk assessment and treatment planning. Of interest, several studies have investigated whether FDG-PET/CT (which is currently routinely performed in DLBCL in many institutions) can replace BMB in DLBCL [Citation16]. However, recent studies in newly diagnosed DLBCL reported that FDG-PET/CT can be negative in up to 30–50% of cases with a positive BMB [Citation14,Citation15]. Moreover, recent studies have shown that, unlike BMB, FDG-PET/CT-based bone marrow status has no prognostic implications [Citation14,Citation15,Citation17], which indicates that FDG-PET/CT cannot replace BMB. Nevertheless, based on the findings of the present study, it is highly questionable whether BMB should routinely be performed at all in newly diagnosed DLBCL, given its minor impact on NCCN-IPI-based risk stratification and lack of treatment consequences.
The present study had several limitations. First, the number of included patients was relatively small. Therefore, the provided data may not be sufficient to directly change the management of DLBCL in this setting, even though the study provides support for this. In addition, the number of included patients with early stage disease was relatively lower than those with advanced stage disease, but this simply reflects the natural distribution of the different disease stages in DLBCL. Second, part of included patients had undergone CT only, whereas the remaining patients had undergone FDG-PET/CT. This is related to the fact that this study spanned the time period between January 2004 and February 2014, and integrated PET/CT only became available for the evaluation of all DLBCL patients at our institution after September 2007. Nevertheless, although the diagnostic performance of CT and FDG-PET/CT may be different, the recently published NCCN-IPI accepts both CT alone and FDG-PET/CT [Citation9]. Third, the prognostic implications of BMB were not formally assessed in this study. However, histologically confirmed bone marrow involvement is already a well-known adverse prognostic factor [Citation7], and has been incorporated into the recently validated NCCN-IPI, the latter which has been used in the present study. Fourth, it should be realized that the results of this study are only applicable to the NCCN-IPI-based risk stratification, the initial start of treatment rather than the entire treatment plan, and the current treatment era in which R-CHOP chemoimmunotherapy plays a pivotal role. BMB may still be of utility in patients who require treatment intensification in case of refractory disease, and who may need pretreatment and follow-up BMB for proper evaluation of response (in case the former is positive for lymphomatous involvement). In addition, treatment guidelines may vary between different institutions, countries, and collaborative groups. Furthermore, in the future, the role of BMB in DLBCL may become more important if more sophisticated risk stratification models and newer (more personalized) treatments become available. Finally, BMB is still a valuable test for the diagnosis of DLBCL if (excisional) biopsy of an extramedullary site is not possible or inconclusive.
In conclusion, although BMB results upstaged the NCCN-IPI-based risk stratification in a small number of cases, this did not have any therapeutic implications in our patient series. These findings support the omission of BMB from routine staging of newly diagnosed DLBCL in the current risk stratification and treatment era.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This project was financially supported by an Alpe d’HuZes/Dutch Cancer Society Bas Mulder Award for T.C.K. (grant number 5409). Data collection, data analysis, and interpretation of data, writing of the paper, and decision to submit were left to the authors’ discretion and were not influenced by Alpe d’HuZes/Dutch Cancer Society. .
References
- Armitage JO. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin Proc 2012;87:161–71.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498–505.
- Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, Andre M, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl 7):vii78–82.
- Vanhelleputte P, Nijs K, Delforge M, Evers G, Vanderschueren S. Pain during bone marrow aspiration: prevalence and prevention. J Pain Symptom Manage 2003; 26:860–6.
- Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy – a review of UK data for 2004. Haematologica 2006;91:1293–4.
- Torlakovic EE, Naresh K, Kremer M, van der Walt J, Hyjek E, Porwit A. Call for a European programme in external quality assurance for bone marrow immunohistochemistry; report of a European Bone Marrow Working Group pilot study. J Clin Pathol 2009;62:547–51.
- Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011;29:1452–7.
- Shim H, Oh JI, Park SH, Jang S, Park CJ, Huh J, et al. Prognostic impact of concordant and discordant cytomorphology of bone marrow involvement in patients with diffuse, large, B-cell lymphoma treated with R-CHOP. J Clin Pathol 2013;66:420–5.
- Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42.
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909–18.
- Lim ST, Tao M, Cheung YB, Rajan S, Mann B. Can patients with early-stage diffuse large B-cell lymphoma be treated without bone marrow biopsy? Ann Oncol 2005;16:215–8.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008.
- Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin 2005;55:368–76. [Review].
- Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Bone marrow F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol 2014;89:726–31.
- Hong J, Lee Y, Park Y, Kim SG, Hwang KH, Park SH, et al. Role of FDG-PET/CT in detecting lymphomatous bone marrow involvement in patients with newly diagnosed diffuse large B-cell lymphoma. Ann Hematol 2012;91: 687–95.
- Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: Systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2014;41:565–74.
- Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 2013;122:61–7.