Abstract
Background. A planning study investigated whether reduced target volumes defined on FDG-PET/CT during radiotherapy allow total dose escalation without compromising normal tissue tolerance in patients with esophageal cancer.
Material and methods. Ten patients with esophageal squamous cell carcinoma (SCC), candidate to curative-intent concomitant chemo-radiotherapy (CRT), had FDG-PET/CT performed in treatment position, before and during (Day 21) radiotherapy (RT). Four planning scenarios were investigated: 1) 50 Gy total dose with target volumes defined on pre-RT FDG-PET/CT; 2) 50 Gy with boost target volume defined on FDG-PET/CT during RT; 3) 66 Gy with target volumes from pre-RT FDG-PET/CT; and 4) 66 Gy with boost target volume from during-RT FDG-PET/CT.
Results. The median metabolic target volume decreased from 12.9 cm3 (minimum 3.7–maximum 44.8) to 5.0 cm3 (1.7–13.5) (p = 0.01) between pre- and during-RCT FDG-PET/CT. The median PTV66 was smaller on during-RT than on baseline FDG-PET/CT [108 cm3 (62.5–194) vs. 156 cm3 (68.8–251), p = 0.02]. When total dose was set to 50 Gy, planning on during-RT FDG-PET/CT was associated with a marginal reduction in normal tissues irradiation. When total dose was increased to 66 Gy, planning on during-RT PET yielded significantly lower doses to the spinal cord [Dmax = 44.1Gy (40.8–44.9) vs. 44.7Gy (41.5–45.0), p = 0.007] and reduced lung exposure [V20Gy = 23.2% (17.3–27) vs. 26.8% (19.7–30.2), p = 0.006].
Conclusion. This planning study suggests that adaptive RT based on target volume reduction assessed on FDG-PET/CT during treatment could facilitate dose escalation up to 66 Gy in patients with esophageal SCC.
The survival probability of esophageal cancer patients remains disappointing [Citation1]. The overall survival is low. Squamous cell carcinoma (SCC) is the most frequent histological type but rising incidences of adenocarcinomas have been reported elsewhere in the world [Citation1]. Chemoradiotherapy (CRT) is the standard treatment of locally advanced or inoperable SCC [Citation1,Citation2]. The proximity of healthy organs often limits the radiotherapy (RT) dose that can be delivered without excessive toxicity [Citation3], possibly hampering tumor control. In 2002, a single randomized trial (INT0123/RTOG94-05) [Citation4] failed to demonstrate a benefit of escalating RT dose from 50.4 to 64.8 Gy using non-conformal RT planning but these date are contradicted by others studies [Citation5]. Since then, the progress in medical imaging (FDG-PET/CT) and RT technique has renewed the interest in dose escalation for esophageal cancer.
Imaging modalities investigating tumor response during CRT are expected to facilitate RT plan adaptation. In case of tumor response, reduced target volumes could receive higher total doses without compromising normal tissue tolerance [Citation6]. FDG-PET/CT is superior to CT scanner to identify the tumor volume in esophageal cancer [Citation7]. When performed during CRT, FDG-PET/CT aims at assessing treatment-induced variations in anatomical and functional tumor volumes that could be used to reduce the RT target volume. In a prospective series [Citation8], 57 patients with esophageal SCC underwent FDG-PET/CT before and during (Day 21) CRT (RTEP3 study, N° NCT 00934505, http://register.clinicaltrials.gov). The functional tumor volumes were smaller at Day 21 of CRT than at baseline FDG-PET/CT [geometric mean (95% confidence limits): 6.5 cm3 (4.7–9.1) vs. 12.8 (9.9–16.7), respectively, p < 10−4]. The patients with high FDG uptake during RCT had a poor outcome.
As an ancillary study to RTEP3, we have investigated whether FDG-PET/CT during CRT would help to plan total doses up to 66 Gy while reducing lung and spinal cord irradiation. We present the results of a planning study performed on a subgroup of 10 patients with FDG-PET/CT acquired in treatment position before and during CRT.
Material and methods
Study design
All patients were included in a prospective study [Citation8] registered to ClinicalTrials.gov Protocol Registration System (http://register.clinicaltrials.gov) in July, 2009 (RTEP3 study, NCT 00934505). To be eligible, the patients had: 1) to have histology-proven esophageal SCC, UICC stage IIB, III or IV (TNM 2002); 2) to be candidate to CRT; and 3) to have given written consent. All patients were to receive CRT according to Herskovic's scheme [Citation9]: continuous RT (1.8–2 Gy per daily fraction, 5 sessions per week, total dose 50–50.4 Gy) and concomitant CT [cis-Platinum 75 mg/m² on Day 1 or 15 mg/m² on Day 1–5, and 5-flurouracil (5FU) 1g/m² continuous IV over 120 hours].
For the present study, we selected a subgroup of patients who underwent FDG PET/CT in treatment position (arms above head for intra-thoracic tumors or along the trunk for cervical tumors, arm or head support depending on arm position, and strict alignment on midline and lateral skin tattoos with laser beams).
FDG-PET/CT imaging
FDG-PET/CT images were acquired while free breathing, at baseline (t0) within 15 days before CRT (PET1) and at Day 21 (± 3 days) during CRT (PET2, see Supplementary Figure 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.973062). The CT scan data were used for attenuation correction and anatomic localization. For each patient, the two FDG-PET/CT were performed on the same machine and under the same acquisition and reconstruction conditions: patients fasting for at least 6 hours, blood-glucose level measured before each FDG-PET/CT, 4.5 MBq/kg of 18FDG injected intra-venously, after a rest of at least 20 minutes, start of acquisition 60 ± 10 minutes post-injection. PET2 acquisition had to be done after the same post-injection interval (± 5 minutes) as for PET1. All PET images were reconstructed using Fourier Rebinning (FORE) and Attenuation Weighted Ordered Subset Expectation Maximization (AWOSEM) algorithm, corrected for random coincidences, scatter and attenuation using the CT scan data, and smoothed with a Gaussian filter (full width at half maximum = 5 mm).
In the present work, a SUV threshold was chosen by two experienced physicians (1 radiation oncologist, 1 nuclear medicine physician) after visual inspection of the region of interest (ROI) and defined an optimal standard uptake value (SUV) threshold (Leonardo workstation, Siemens Medical Solutions, Hoffman Estates, Knoxville, TN, USA). This process allowed excluding FDG uptake due to treatment-induced esophagitis. This approach was used on a larger series of 66 consecutive patients imaged in our institution [Citation10]. The concordance between investigators was validated on a subset of 26 patients. Our 10 patients were abstracted from this series according to the following criteria: 1) inclusion in the RTEP3 prospective study; and 2) FDG PET/CT acquisition in RT position. One should note that such an approach requires strict quality insurance to obtain: 1) comparable SUV values (i.e. standardization of the acquisition process); and 2) reliable co-registration of sequential acquisitions (i.e. PET/CT in treatment position). In a second time, CT1 and CT2 were automatically registered on vertebrae (with manual adjustment if necessary) so that the delineated volumes could be superimposed and transferred to the RT planning workstation.
The FDG uptake volumes delineated on PETs were considered as gross tumor volume (GTVPET). Primary tumor and positive mediastinal lymph nodes were labeled as GTVtPETn and GTVnPETn respectively, n being either 1 (PET1 at baseline) or 2 (PET2 at Day 21 during CRT). The GTVPET included the primary tumor (GTVtPET) and the nodes (GTVnPET) delineated on the same PET. SUVmax were measured in each GTVPET. The nodes with small diameter > 1 cm on CT were included in the GTV.
To account for microscopic disease extension, a clinical target volume (CTV) was defined by adding a 5-cm cranio-caudal margin to the GTVPET, then manually excluding the anatomical barriers (lung, trachea, large vessels, bones and heart) when not involved. Any mediastinal lymph node station containing nodal GTV or with a risk of invasion ≥ 20% was delineated as part of the CTV. This CTV was to receive 40 Gy (CTV40) in all plans.
Four different plans (i.e. scenarios) were calculated for each patient:
| 1) | Plan 1: 50-Gy total dose to a CTV50 extending 2 cm superiorly and inferiorly from GTVtPET1 excluding the anatomical barriers, plus the lymphnode stations with FDG uptake at PET1, | ||||
| 2) | Plan 2: 50-Gy total dose to a CTV50 extending 2 cm superiorly and inferiorly from GTVtPET2 excluding the anatomical barriers, plus the lymphnode stations with FDG uptake at PET2, | ||||
| 3) | Plan 3: 66 Gy to a CTV66 with the same cranio-caudal margins around the GTVtPET1 and a 5-mm isotropic margin around FDG positive lymphnodes at PET1 (manually edited to exclude anatomical barriers). | ||||
| 4) | Plan 4: 66 Gy to a CTV66 with the same cranio-caudal margins around the GTVtPET2 and a 5-mm isotropic margin around FDG positive lymphnodes at PET2 (manually edited to exclude anatomical barriers). | ||||
The four corresponding PTVs were obtained by adding a 7 mm isotropic margin around the CTVs (see Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.973062).
Planning
The plans were based on four beams conformed on Beam's Eye Views to the sequential PTVs. Eighteen MV X beams were used, except for cervical tumors where 6MV X rays were allowed if necessary. The doses were calculated using an ECLIPSE treatment planning system (VARIAN® v10.0) without heterogeneity correction.
The doses to the PTVs were prescribed at the isocenter. The objectives for PTV doses were minimal and maximum doses between 95% and 107% of the prescribed dose, and 95% of the PTV included in the 95% isodose curve. To compare the four scenarios, we used the doses received by 98%, 50% and 2% of the PTVs, respectively termed as D98, D50 and D2. The constraints for the organs at risk (OAR) were: lungs: V20Gy < 27% and V30Gy < 20% of the total lung volume (including the PTVs); spinal cord: maximum dose < 45Gy; heart: V40Gy < 30%; liver: V30Gy < 40%; kidneys: V20Gy < 25%.
Population
Between May 2009 and April 2011, 57 patients were prospectively included in the RTEP3 study (see details in [Citation8]). The records of 13 patients with FDG-PET/CT's performed in RT position were selected for the present analysis. The tumor sites were eight (57%) middle esophagus, four (29%) upper esophagus and two (14%) lower esophagus, patient #32 having two primaries. The tumor stages were seven (50%) T3N0, six (43%) T3N1 and one (7%) T4N1 (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.973062).
The baseline FDG-PET/CT (PET1) was performed at a median time interval of 14 days (range 3–42 days) before the start of RCT and PET2 at median Day 21 (range 18–23 days) of RCT.
Statistical analysis
The objective was to investigate whether the target volumes could be irradiated to either 50 Gy (plans 1 and 2) or 66 Gy (plans 3 and 4). The parameters (Dmax, Vdose) describing the OARs’ dose distributions were compared using a non-parametric test for quantitative variables (Wilcoxon signed-rank test for difference in medians with approximation for continuity correction, NCSS 2007 version 07.1.18, Kaysville, UT, USA). A difference was considered as statistically significant if p < 0.05 (two-sided tests).
Results
All patients had a significant FDG uptake on PET2. The GTVPET2 could not be reliably delineated in three patients (#10, #47 and #54) with acute esophagitis on PET during RT. In the 10 remaining patients, the median GTVPET decreased from 12.9 cm3 (minimum 3.7–maximum 44.8) to 5.0 cm3 (1.7–13.5) (p = 0.01) between PET1 and PET2 (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.973062).
The comparison of the four different PTVs is shown on . The differences between the median volumes of PTV50-1 and PTV50-2 were close to statistical significance [215 cm3 (102–459) vs. 182 cm3 (94.4–343) at PET1 and PET2, respectively, p = 0.053]. The PTV66 was smaller on PET2 than on PET1 [156 cm3 (68.8–251) vs. 108 cm3 (62.5–194), p = 0.02].
Figure 1. PTV volumes in the four scenarios. PTV40: PTV to receive 40 Gy in all scenarios; PTV50-1 and PTV50-2: PTV to receive 50 Gy derived from PET1 (pre-RCT) and PET2 (during-RCT); PTV66-1 and PTV66-2: PTV to receive 66 Gy derived from PET1 and PET2.
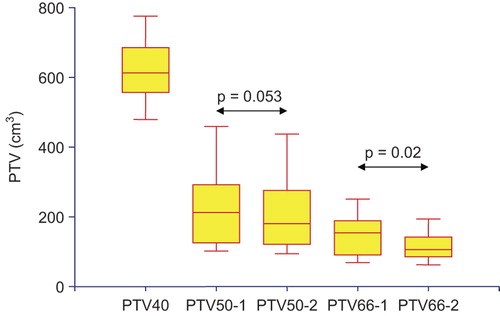
shows the comparisons between scenarios 1 and 2, i.e. when planning a total dose of 50 Gy in the PTV50. No significant differences were observed in the respective PTVs’ coverage. Planning on PET2 was associated with a significant reduction in lung V20, although the absolute gain was small [-0.15% (−1.1–0.1), p = 0.03]. The reduction in lung V30 was close to significance (p = 0.0503). Large absolute benefits in heart V40, liver V30 and esophagus DMax were observed occasionally, although statistical significance was not reached.
Table I. Planning up to 50 Gy on PET1 (scenario 1) versus on PET2 (scenario 2). Figures are medians [Minimum–Maximum]; ∆(PET2–PET1): difference between the values obtained when planning on PET2 and PET1; p: p-values for Wilcoxon signed-rank test for difference in medians (approximation with continuity correction, two-sided).
When aiming at total doses of 66 Gy (scenarios 3 and 4, ), planning on PET2 was associated with a significant reduction in the dose received by 2% of PTV66 [D2, median -0.45Gy (-1.4–0.0), p = 0.007], suggesting less heterogeneous dose distributions in the boost target volume. Significant differences in favor of planning on PET2 were observed for spinal cord DMax, lung V20 and V30, heart V40 and liver V30. The largest absolute benefits were observed for lung V20 [median -2.15% (-5.4–0.2), p = 0.06] and heart V40 [median -1.8% (-7.1–2.2), p = 0.047], while the individual values could be much higher (i.e. -5% in lung V20 and V30, -7 and -5% in heart V40 and liver V30).
Table II. Planning up to 66 Gy on PET1 (scenario 3) versus on PET2 (scenario 4). Same abbreviations as in .
Discussion
The present planning study was conducted on 10 pairs of pre- and during-RCT FDG-PET/CT out of 57 patients with esophageal SCC included in a prospective study on the prognostic value of FDG-PET/CT during CRT [Citation8]. This is the first report investigating adaptive planning taking into account tumor metabolic response at Day 21 of CRT. The total RT dose could be increased from 50 to 66 Gy while significantly reducing normal tissue exposure. When the dose was left to 50 Gy, the benefit of replanning on PET during CRT did not reached statistical significance in terms of OARs’ dose distribution. Further clinical studies are necessary to evaluate whether there is any benefit of this approach in terms of local control and survival.
The analysis of the whole RTEP3 study group demonstrated that the functional tumor volumes measured on FDG-PET/CT at Day 21 of CRT were significantly smaller than on baseline PET/CT [Citation8]. This observation prompted us to investigate whether tumor response could yield improved dose distributions after replanning, adapting the RT technique to the reduced target volumes. The patients having had their FDG PET/CT acquired in treatment position were selected for reliable image sets registration and dose calculations. The dose calculations were not corrected for tissue heterogeneities since our PET/CT devices had not been calibrated: Hounsfield units could not be converted into electronic densities. The different steps of the study were conducted in a single center by the same team (radiation oncologists, nuclear medicine physicians, medical physicists). The target volumes and OAR delineation procedures, as well as the planning objectives and dose/volume constraints were those for daily use in our department and recommended for centers participating in the CONCORDE prospective trial (see below). Contrasting with the large reduction in PTV on PET2, the median reduction in OAR irradiation was moderate, although statistically significant when increasing total dose to 66 Gy. Larger benefits were reported with IMRT [Citation6,Citation11–13]. For instance, Kole et al. [Citation12] compared the plans obtained with 3D-RT and IMRT to deliver 50.4 Gy in 19 patients with distal esophageal cancer and reported that the mean dose to the heart was reduced from 28.2 to 22.9Gy (p < 0.05). Welsh et al. [Citation6] showed on 10 patients that 66.4 Gy could be delivered with simultaneous integrated boost (SIB) IMRT while reducing the dose to the normal heart, lung, and liver when compared to 50.4 Gy with a 2D-CRT plan. IMRT for thoracic tumors is not routinely available in our institution and we decided to stick to the recommendations of a recently launched French trial (Radiochemotherapy With and Without Dose Escalation in Patients Presenting Locally Advanced or Inoperable Carcinoma of the Oesophagus: CONCORDE, ClinicalTrials.gov Identifier: NCT01348217) comparing 50 Gy versus 66 Gy RCT.
FDG-PET/CT has an established role in the diagnosis and staging of esophageal cancer [Citation14], although its use for RT planning is still debated [Citation15]. As for the detection of metastatic mediastinal lymph nodes, the sensitivity ranges between 30% and 93% and the specificity between 79% and 100% [Citation15]. We included in the GTV the nodes with small diameter > 1 cm on CT to avoid the potential consequences of false negative FGD-PET/CTs [Citation16]. As for the primary tumor, the delineation on a sole planning CT has been criticized [Citation15,Citation16]. However, no standard method has yet been accepted to delineate the primary tumor on FDG-PET/CT. When comparing tumor lengths as measured on FDG-PET/CT and on pathological specimens, fixed SUV threshold values of 2.5 [Citation17] or 1.4 [Citation18] have been proposed, although CT and FDG PET/CT appeared to be complementary. In non-small cell lung cancer (NSCLC), when delineating a tumor volume during RT or CRT, the manually drawn volumes seemed to be more reliable than the volumes delineated by automatic or semi-automatic methods [Citation19].
Relatively high (around 50% in the CROSS trial [Citation20] pathological response rates have been reported after moderate doses of RT [Citation20,Citation21]. However, several arguments support the potential of increased RT dose in esophageal cancer. Several randomized trials reported local-relapse rates around 40–50% after RCT alone [Citation4,Citation22] while 90% of the local relapses occurred in the GTV in a retrospective series [Citation4]. Randomized trials comparing RCT alone versus RCT plus surgery demonstrated that surgery improved loco-regional control, although not associated to increased overall survival probability [Citation22]. This suggests that a more intensive treatment (i.e. RCT plus surgery or escalated RT dose) would improve loco-regional control. In a single randomized trial (INT0123/RTOG94-05) [Citation4], 218 patients received concomitant CRT and RT doses of either 64.8 Gy or 50.4 Gy. After a median follow-up duration of 29.5 months in surviving patients, no significant benefit of higher RT doses was observed in terms of overall survival or local/regional control. The trial was opened in 1995 and closed in 1999, so that treatment planning was based on classical simulator fields. It may well be assumed that conformal techniques based on planning CT (and more recently on FDG-PET/CT) would have achieved a more accurate coverage of the target volumes [Citation6]. In NSCLC patients, two phase I–II studies have suggested a benefit of higher RT doses [Citation23,Citation24]. The increased dose levels were selected so that lung tolerance would not be impaired. Smaller tumors, i.e. situations where lung exposure was already limited, received higher doses. Therefore, the reported correlations between higher doses and better control rates may also reflect a spurious link between total dose and tumor size. Combined analyses of head and neck cancers in retrospective series were consistent with a 2% increase in tumor control when RT dose is increased by 1% [Citation25]. The clinical demonstration requires both accurate dosimetry and delivery (not achieved according to nowadays criteria in the INT 0123) and a population of tumors that are homogeneous regarding their radiosensitivity [Citation25]. The ongoing efforts with IMRT [Citation8,Citation15–18,Citation20], IMRT with protons, along with the CONCORDE trial, demonstrate that dose escalation in esophageal cancer is still a domain of active clinical research.
The adaptive strategy presented here deserves more investigation before FDG-PET/CT during CRT can be implemented in routine. Kwee et al. [Citation21] reported sensitivity and specificity values ranging from 33% to 100% and from 30% to 100%, respectively, when FDG PET/CT was used to predict pathological response to RCT. Technically speaking, the low resolution of PET images, possibly results in apparent response while viable tumors cells still exist. A counter argument is that those viable cells could be much less numerous in areas associated to reduced FDG uptake, therefore requiring less RT total dose. These areas are likely to lie in the vicinity of the residual volume and to be included in the CTV for dose increase (5 mm lateral/2 cm cranio-caudal margins in the present study).
Although statistically significant, the median reduction in dose to the OARs may be considered as making limited clinical sense. Our data suggest that not all patients would benefit from replanning on FDG-PET/CT during RT. Given the limited size of our sample, we could not identify these patients. In the CONCORDE trial, FDG-PET/CT will be repeated around 50 Gy, hopefully providing new data to assess the prognostic/predictive value of FDG-PET/CT and its role in the determination of RT target volumes. In the mean time, FDG-PET/CT during RCT for esophageal cancer should be considered as investigational.
In summary, FDG-PET/CT performed at Day 21 of CRT for esophageal cancer shows a decrease in metabolic tumor volume. Our planning study indicates that adapting the target volumes on the basis of FDG-PET/CT images acquired during treatment could facilitate an increase in total RT dose within the routine frame of normal tissues dose/volumes constraints, associated to better sparing of the spinal cord and lungs.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.973062.
ionc_a_973062_sm3083.pdf
Download PDF (86.2 KB)Declaration of interest: The authors thank David Voisard (radiation oncology and Medical Physics, Henri Becquerel Center) for his help in developing this article. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381:400–12.
- Pottgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer – a meta-analysis of the randomized trials. Cancer Treat Rev 2012;38:599–604.
- Morota M, Gomi K, Kozuka T, Chin K, Matsuura M, Oguchi M, et al. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 2009;75:122–8.
- Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74.
- Hurmuzlu M, Øvrebø K, Wentzel-Larsen T, Muren LP, Viste A, Smaaland R. High-dose preoperative chemoradiotherapy in esophageal cancer patients does not increase postoperative pulmonary complications: Correlation with dose-volume histogram parameters. Radiother Oncol 2010;97:60–4.
- Welsh J, Palmer MB, Ajani JA, Liao Z, Swisher SG, Hofstetter WL, et al. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys 2012;82:468–74.
- Lambrecht M, Haustermans K. Clinical evidence on PET-CT for radiation therapy planning in gastro-intestinal tumors. Radiother Oncol 2010;96:339–46.
- Palie O, Michel P, Menard JF, Rousseau C, Rio E, Bridji B, et al. The predictive value of treatment response using FDG PET performed on day 21 of chemoradiotherapy in patients with oesophageal squamous cell carcinoma. A prospective, multicentre study (RTEP3). Eur J Nucl Med Mol Imaging 2013;40:1345–55.
- Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. New Engl J Med 1992;326: 1593–8.
- Lemarignier C, Di Fiore F, Marre C, Hapdey S, Modzelewski R, Dubray B, et al. Eur J Nucl Med (in press).
- Chandra A, Guerrero TM, Liu HH, Tucker SL, Liao Z, Wang X, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005;77:247–53.
- Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580–6.
- Welsh J, Gomez D, Palmer MB, Riley BA, Mayankkumar AV, Komaki R, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: A dosimetric study. Int J Radiat Oncol Biol Phys 2011;81:1336–42.
- van Westreenen HL, Westerterp M, Bossuyt PM, Pruim J, Sloof GW, van Lanschot JJ, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 2004;22:3805–12.
- Muijs CT, Beukema JC, Pruim J, Mul VE, Groen H, Plukker JT, et al. A systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancer. Radiother Oncol 2010;97:165–71.
- Vrieze O, Haustermans K, De Wever W, Lerut T, Van Cutsem E, Ectors N, et al. Is there a role for FGD-PET in radiotherapy planning in esophageal carcinoma? Radiother Oncol 2004;73:269–75.
- Mamede M, El Fakhri G, Abreu-e-Lima P, Gandler W, Nose V, Gerbaudo VH. Pre-operative estimation of esophageal tumor metabolic length in FDG-PET images with surgical pathology confirmation. Ann Nucl Med 2007;21:553–62.
- Han D, Yu J, Yu Y, Zhang G, Zhong X, Lu J, et al. Comparison of (18)F-fluorothymidine and (18)F-fluorodeoxyglucose PET/CT in delineating gross tumor volume by optimal threshold in patients with squamous cell carcinoma of thoracic esophagus. Int J Radiat Oncol Biol Phys 2010; 76:1235–41.
- Edet-Sanson A, Dubray B, Doyeux K, Back A, Hapdey S, Modzelewski R, et al. Serial assessment of FDG-PET FDG uptake and functional volume during radiotherapy (RT) in patients with non-small cell lung cancer (NSCLC). Radiother Oncol 2012;102:251–7.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New Engl J Med 2012;366:2074–84.
- Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: A systematic review. Radiology 2010;254: 707–17.
- Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
- Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2012;82: 425–34.
- van Baardwijk A, Bosmans G, Boersma L, Wanders S, Dekker A, Dingemans AM, et al. Individualized radical radiotherapy of non-small-cell lung cancer based on normal tissue dose constraints: A feasibility study. Int J Radiat Oncol Biol Phys 2008;71:1394–401.
- Bentzen MS. Dose-response relationships in radiotherapy, In: Basic clinical radiobiology, 4th ed. Joiner M, van der Kogel A, editors. London: CRC Press; 2010. pp 56–66.
