Abstract
Background. Treatment of localized prostate cancer (PC) is controversial. This is the first randomized study comparing an open surgery procedure (radical prostatectomy) with a combination of high-dose rate brachytherapy (2 × 10 Gy) and external beam radiotherapy (25 × 2 Gy) in PC patients in Sweden 1996–2001. The two randomization arms were compared regarding differences in patients-reported outcomes, such as complications and health-related quality of life (HRQoL).
Material and methods. The patients had localized/locally advanced PC, clinical category T1b–T3a, N0, M0 and PSA ≤ 50 ng/ml. All underwent total androgen blockade (six months). Self-reported HRQoL and symptoms including urinary, bowel, and sexual side effects were investigated prospectively before randomization and 12 and 24 months after randomization. A total of 89 patients were randomized and completed the EORTC QLQ C-33 and EORTC PR-25 questionnaires.
Results. Over the study period, there were no discernible differences in HRQoL, or complications between the two groups. Emotional functioning, however, improved statistically significantly over time, whereas Social functioning decreased, and financial difficulties increased. No statistically significant differences in group-by-time interactions were found. The survival rate was 76%. Only eight patients (9%) died of PC.
Conclusion. Open radical prostatectomy and the combined high-dose rate brachytherapy with external beam radiation appeared to be comparable in the measured outcomes. It was not possible to draw any conclusion on the efficacy of the two treatments due to insufficient power of the study.
Prostate cancer (PC) is the most widespread form of cancer among men in Sweden, with an annual incidence of approximately 9000 new cases [Citation1]. Curative-intended treatments for localized/locally advanced disease include radical prostatectomy (RP) and radiotherapy (RT). Only a limited number of randomized studies have, however, compared these treatment options [Citation2]. Long-term survival after PC is common also without therapy [Citation3]. The optimal treatment is still a matter of debate. Important aspects to consider in that the treatment decision process include differences in side effects and complications, as well as the impact on health-related quality of life (HRQoL). To our knowledge, no randomized studies in the literature have yet been published comparing high-dose rate brachytherapy with EBRT and surgery as primary treatment for PC.
The Protect T study, conducted in the UK, randomized men diagnosed with clinically localized PC to RT, RP, or active monitoring. So far, however, only baseline data have been published [Citation4]. One meta-analysis found hormone suppression combined with RT significantly decrease recurrence and mortality in localized PC, without affecting toxicity [Citation5]. Another systematic review and meta-analysis of randomized trials of neo-adjuvant hormone therapy prior to prostatectomy did not show improved overall or disease-free survival, but did reveal reduced positive margin rates, organ confinement, and lymph node invasion [Citation6]. In the SPCG-7 randomized trial, which included patients with locally advanced or high-risk localized PC reported that adding RT to endocrine treatment decreased the 10-year PC- specific mortality by 50%, and substantially decreased overall mortality [Citation7]. Interestingly, no substantial differences in HRQoL between patients treated with RP and those subjected to high-dose rate (HDR) brachytherapy, was reported by Jo et al. [Citation8], although the patients with the latter treatment did report better urinary and sexual functions.
This paper is the first report on a study, performed in Sweden 1996–2001, in which patients with localized/locally advanced PC were randomized to HDR brachytherapy (the RT group) (2 × 10 Gy) combined with external beam RT (EBRT, 25 × 2 Gy) or to an open surgery procedure (the RP group). The aim was to assess differences between the two treatment arms with regard to patient-reported outcomes, such as complications and HRQoL.
Material and methods
The study was randomized, parallel and open. Men in Sweden who had localized/locally advanced PC clinical category T1b–T3a, N0, M0 (UICC, TNM, 1992) and a PSA value ≤ 50 ng/ml were included in 1996–2001. PC was proven histopathologically by ultrasound-guided transrectal core-needle biopsy, mapping a total of six biopsies from all four quadrants and at least two biopsies from the base of the seminal vesicles. Bone scans were performed on all patients with a PSA level ≥ 10 ng/ml and not older than three months at randomization. Patients should have accepted RP or RT and possible to perform. Patients should not have gone through myocardial infarction within the last six months; serum bilirubin, ASAT/ALAT should not exceed 1.2 times normal highest reference limit. Other malignant disease, excluding basal cell carcinoma, was an exclusion criteria. Patients should be capable to follow study rules. The five participating centres were located in Gothenburg, Uppsala, Linköping, Eskilstuna, and Stockholm. Before inclusion, the patients were given full oral and written information about the study and the respective treatment modalities, and provided written informed consent. The study was approved in March 1996 by the Regional Ethics Committee, Gothenburg, Sweden (No. 44/96).
Randomization
The patients were randomized to HDR brachytherapy (2 × 10 Gy) combined with EBRT (25 × 2 Gy) (the RT group) or to an open surgery procedure (the RP group). Randomization was performed by telephone and recorded at a central registration office at the Regional Oncology Centre, Sahlgrenska Hospital, Gothenburg. The patients were stratified according to the following: treating centre; G1–G2 or G3, and T1–T2 or T3; age < 70 years or ≥ 70 years; PSA < 20 or ≥ 20 ng/ml.
Treatment regimens
All patients were treated with total androgen blockade (TAB), consisting of a combination of anti- androgen and gonadotropin releasing hormone (GnRh) analogue in the neo-adjuvant setting. The TAB included leuprorelin (s.c. 3.75 mg every 4th week) and flutamide (250 mg orally three times a day) that continued for six months.
Prostatectomy (the RP group)
Patients randomized to RP underwent lymph node evaluation in connection to surgery. Only node- negative patients proceeded to a nerve sparing RP, which was performed within 3–4 months after randomization. Lymphadenectomy was conducted in patients with stage T1b-T2 PC and PSA ≥ 20 ng/ml and in all those with either T3 tumors, irrespective of grades, or grade 3 tumors irrespective of stages. Bilateral lymph node dissection was done with the recommended laparoscopic technique described by Grenabo et al. [Citation9] with bilateral node dissection including obturator nodes. The recommended RP procedure was the nerve sparing method described by Walsh et al. [Citation10]. The surgeon aimed to conduct a radical operation and sacrificed the neurovascular bundles on the tumor side. If the patient was found to have more extensive disease than presumed preoperatively, surgery was still performed if technically feasible.
EBRT and HDR brachytherapy (the RT group)
Irradiation given as a combination of EBRT and HDR brachytherapy was initiated within 3–4 months after randomization. Before that, they all had lymph node dissection according to above described criteria.
EBRT
The clinical target volume (CTV) comprised the tumor and the entire prostate gland with a margin of 0.5 cm. The planning target volume (PTV) included CTV with a margin of 1.5 cm. If the posterior extension of this margin included more than half of the rectal lumen, the margin in this direction was restricted to encompass less than half of that area. RT was planned with a three-dimensional (3D) dose planning system (Dosetech or Helax), delivered with at least 8 MV photon beams.
HDR brachytherapy
CTV comprised the entire prostate including the tumor. PTV included an additional 3-mm margin. The minimum radiation dose was 10 Gy. The recommended rectal dose was not to be given in excess of 6 Gy, defined as the dose to the rectal volume outside a 3-cm long line drawn parallel to the dorsal limitation of the prostate. Two brachytherapy treatments given at a two-week intervals were planned for each patient. If the first brachytherapy session caused toxicity, or if the patient did not participate in a second session for any reason, the second treatment session was replaced with additional external RT of 14 Gy. All patients were evaluated according to the intention-to-treat principle.
Follow-up
Patients were clinically evaluated six and 12 months after randomization, every six months during the second year, and thereafter once a year. Digital rectal examination was performed if serum PSA was ≥ 10 ng/ml. Follow-up assessments at 12, 24, and 60 months were supplemented with the RTOG/EORTC toxicity scale, mailed to the home addresses of the patients. Additional hormone therapy was given according to local routines. Postoperative RT was not allowed according to the protocol.
Survival data
In 2011, included patients who died were identified in the Swedish death registry. The medical records of the deceased patients treated at the respective centres were reviewed to ascertain causes of death.
HRQoL assessment
HRQoL was assessed on three occasions: before randomization to therapy and 12 and 24 months after randomization. The physician or study nurse gave each patient the first questionnaires, which were to be completed before randomization. On the subsequent assessment occasions, the questionnaires and prepaid return envelopes were sent to the patients’ home address from the Department of Oncology, Sahlgrenska University Hospital, Gothenburg.
The European Organization of Research and Treatment of Cancer Quality of Life Questionnaire C33 (EORTC QLQ-C33) was developed for measurement of HRQoL in cancer patients participating in clinical trials [Citation11]. This version of the instrument was available at the start of the present study, a version later developed to the EORTC QLQ-C30.
The EORTC QLQ-C33 comprises 33 items incorporating five single-item scales and nine multi-item scales evaluating the following function (physical, role, cognitive, emotional, and social dimensions), symptoms (fatigue, pain, nausea/vomiting, sleeping problems, constipation, appetite loss, dyspnea, diahorrea), financial problem as well as global health and QoL.
A PC-specific HRQoL questionnaire consisting of 20 items was developed in Gothenburg, Sweden, to gather information on specific problems experienced by PC patients with respect to bowel, urinary tract, and sexual functions. This was the initial version of the questionnaire EORTC PR-25.
Statistics
At the start of the current study, it was estimated that it would be necessary to randomize 360 patients to the two assessment groups in order to detect a difference in survival of 15% and 30%, respectively (Fisher's exact test, two-tailed at the 0.05 level). However, due to the low number of patients actually included (n = 89), evaluation of survival could not be conducted. However, the Swedish death registry was used to identify number of deaths. The records of all patients were carefully checked to avoid misclassification.
Mean values and standard deviations for the patient-reported outcomes recorded on each assessment occasion are given separately for the two randomization groups. Missing values were substituted according to the EORTC QLQ scoring manual [Citation12]. ANOVA repeated measurements were used to evaluate differences between assessment occasions in each group.
The level of statistical significance was set to ≤ 0.01 due to multiple comparisons. The results were evaluated according to the intention-to-treat principle.
Amendments
There were three amendments to the study protocol. The first, November 1996, ended specified inclusion of lymph node staging during prostatectomy. The second amendment, March 1998, entailed addition of the erectile dysfunction. The third amendment, July 2001, stipulated that only T1–T2 tumors and PSA < 20 ng/ml without TAB could be included, and that a bone scan and staging should be performed in patients with G3 tumors. No patient was included according to the third amendment before closing the study.
Results
A total of 89 patients were included in the study and randomized. presents the characteristics of the patients. There were, no statistically significant differences between the two randomization groups. One of the patients randomized to RT underwent EBRT only and got 70 Gy, and thus no brachytherapy. As it was difficult to include patients after the third amendment, it was decided to close the study in April 2002.
Table I. Clinical characteristics and age according to randomization group.
Patient-reported outcomes
Before randomization, questionnaires were completed by 67 patients (75%): 36 (80%) in the RP group and 31 (71%) in the RT group. Corresponding figures for the 12-month assessment were 76 patients (85%): 39 (87%) and 37 (84%), respectively, in the two groups. A total of 76 patients (85%) completed the 24-month assessment: 38 (84%) in the RP group and 38 (86%) in the RT group. A total of 59 patients (66%) completed the questionnaires on all three assessment occasions.
Health-related quality of life (HRQoL)
Mean values and standard deviations for the two randomization groups on the three assessment occasions are presented in . No statistically significant differences between the two randomization groups were found for any of the HRQoL variables. There was a statistically significant improvement in emotional functioning over time (df = 2.57, F = 8.227, p = 0.0005). Also, social functioning decreased with time (df = 2.57, F = 5.540, p = 0.0051), and financial difficulties increased (df = 2.57, F = 7.225, p = 0.0011). We found no statistically significant group-by-time interactions. Furthermore, there were no significant differences at the time of randomization between those who completed all three assessments and those who did not with respect to any of the HRQoL variables.
Table II. Mean scores and standard deviations (SD) for the EORTC QLQ-C33 subscales and single items at three points of assessment according to randomization arm.
Complications
presents the proportions of patients in the two randomization groups reporting PC-specific problems in each response category on the three assessment occasions. No statistically significant differences were found between the groups. A statistically significant group-by-time interaction was found for urinary incontinence (df = 55,2; F = 7.304; p = 0.0011). Grade 4 urinary incontinence was not reported in the RT group at the one-year assessment, whereas 8% (n = 3) had this problem in the RP group. At the two-year assessment, grade 3–4 urinary incontinence was reported by 10% (n = 3) in the RT group compared to 16% (n = 4) in the RP group. Also, fecal incontinence after two years was experienced by 24% (n = 7) in the RT group compared to 8% (n = 2) in the RP group. Both groups reported diminished sexual interest (df = 53,2; F = 11.789; p < 0.0001) and erectile dysfunction (df = 52,2; F = 49.77; p < 0.0001). In addition, 86% (n = 29) of men in the RT group and 90% (n = 33) in the RP group reported having erectile dysfunction of grade 3–4 after two years.
Table III. Frequencies of prostate cancer-specific problems, patients-reported response categories according to randomization group at three points of assessment.
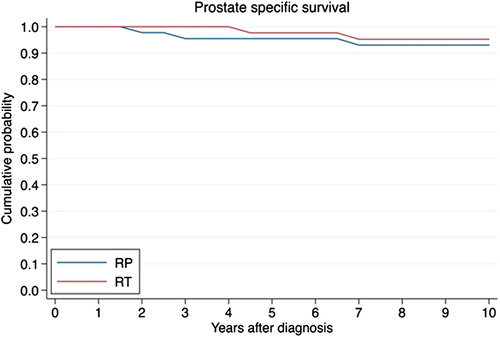
Survival
A total of 68 patients (76%) were still alive in 2011, 10 years after the last patient was randomized into the trial. Survival curves for the two randomization groups are presented in . Eight patients (9%) (n = 6 in RP-group and n = 2 in RT-group) died of PC, and 13 patients died (n = 6 in RP-group and n = 7 in RT-group) of other causes.
Discussion
The management of patients with localized PC is controversial. The treatment options range from initial aggressive therapy (e.g. various forms of radiation therapy or radical surgery) to active surveillance. To our knowledge, the current study represents the first report of a prospective randomized study comparing prostatectomy to a combination of radiotherapy (HDR brachytherapy + EBRT). Although Paulson DF [Citation13,Citation14] randomized patients to compare RP with external beam radiotherapy, no conclusion could be drawn in that study due to heterogenicity of study population. The present randomized trial was performed between 1996 and 2001 and included Swedish men with localized/locally advanced PC. The aim, at the start of the study, was to examine differences in survival and also patient-reported outcomes including HRQoL, symptoms in the urinary tract and bowel, and sexual disturbance before, during, and after treatment. The study, however, became underpowered as it was closed before the intended number of patients had been reached. Clearly, this precludes any conclusions to be drawn on the survival and progression data, which formed the basis for the intended sample size. There were several reasons for the low inclusion rate. One reason was that two other competing studies (the SPCG-4 and SPCG-7 trials) recruited PC patients at the same time. Further, the addition of hormone therapy in the surgery group probably affected physicians willingness to include in this study, as it was reported in 1998 (during this study period) that there were no difference in patients progression-free survival in prostatectomy alone compared to surgery with added neo- adjuvant endocrine treatment [Citation15]. Although, the third amendment to the study protocol in 2001 excluded neo-adjuvant endocrine treatment, still no patient was randomized after that time point, and thus it was decided to close the study.
HRQoL was investigated in a Swedish study in PC patients 22 months after brachytherapy [Citation16]. Dysuria was reported by 4%, frequency (≥ 1 voiding/hour) by 5%, hematuria by 12%, and daily incontinence by 5%. Although different questionnaires were used in the cited study, the results indicate that urinary problems are more common in men after RP than after RT, and these problems increase over time. In our study, grade IV urinary urgency was 3% in both groups at randomization and also at the 24-month assessment. Furthermore, there was no difference in grade 4 urinary incontinence between the groups at the 24-month assessment. Fecal incontinence seemed to be more common after RT. Bowel problems were also relatively common in the previous mentioned Swedish study, where 34% of the patients reported that problem [Citation16]. Grade I rectal bleeding also appeared to be a larger problem in the RT group than in the RP group in our study. These problems are probably due to late side effects of RT.
Both of the present treatment groups reported major sexual problems at 24-months: 92% in the RP group and 86% in the RT group. However, 68% of the patients in the study by Wahlgren et al. [Citation16] reported sexual problems approximately two years after the start of RT. This discrepancy can probably be explained by a treatment effect. Age might be an additional explanation. The chance for preserving erectile function seemed better if the patient was younger and had a good erectile function before treatment [Citation16].
We detected no statistically significant differences between the two randomized groups with regard to any of the HRQoL variables. Over time, there was a statistically significant improvement in emotional functioning, whereas social functioning decreased and financial difficulties increased. HRQoL levels in our study were comparable to those observed in a prospective study of PC patients after brachytherapy and EBRT [Citation17] in which no differences in HRQoL were found between the patients and a normative sample of Swedish men. The levels of HRQoL noted in our investigation correspond to those found in previous studies supporting the validity of our results. One explanation for the decrease in social functioning could be the bowel and urinary problems reported after PC treatment.
Despite that the current study did not reach its intended size, it has strengths. First, to our knowledge, it represents the only randomized study comparing prostatectomy with combined HDR irradiation and EBRT. Second, HRQoL was assessed prospectively by use of the EORTC QLQ-C33, which is a standardized validated questionnaire developed in international collaboration, and third, the response rate was high.
Conclusions
This randomized study showed no statistically significant differences in HRQoL and complications between patients subjected to RP and those given high-dose rate brachytherapy combined with external beam radiation therapy. Few patient died during the 10-year follow-up, but no conclusions can be drawn regarding differences in survival as the study was underpowered.
Acknowledgments
This study was supported by grants from the following sources in Sweden: the King Gustav V Jubilee Clinic Cancer Research Foundation in Gothenburg, the Swedish Cancer Society, the Cancer Society Jubilee Fund in Stockholm, and Schering Plough (Owe Nylander). The authors also thank B. J. Norlén, G. Borghede, and H. Hedelin for promoting the study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cancer incidence of Sweden, Centre for Epidemiology. National Board of Health and Welfare: Stockholm; 2009.
- Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer Results Study Group. Br J Urol Int 2012:109;s1.
- Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localised prostate cancer. JAMA 2005;293:2095–101.
- Jane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: CAP and Protec T studies. Eur J Cancer 2010;46:3095–10.
- Bria E, Cuppone F, Giannarelli D, Milella M, Ruggeri EM, Sperduti I, et al. Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer? Cancer 2009;115:3446–56.
- Shelley MD, Kumar S, Wilt T, Staffurth J, Coles B, Mason MD. A systematic review and meta-analysis of randomized trials of neo-adjuvant hormone therapy for localized and locally advanced prostate carcinoma. Cancer Treat Rev 2009;35:9–17.
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment with or without radiotherapy in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomized phase III trial. Lancet 2009;373:301–8.
- Jo Y, Junichi H, Tomohiro F, Yoshinari I, Masato F. Radical prostatectomy versus high-dose rate brachytherapy for prostate cancer: Effects on health-related quality of life. Br J Urol Int 2005;96:43–7.
- Grenabo L, Grundtman S, Hedelin H. Laparoscopic obturator lymph node dissection in patients with prostate cancer. Scand J Urol Nephrol 1995;29:51–5.
- Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol 1984; 56;694–7.
- Aaronson NK, Ahmedzai S, Bergman B. The European Organization for Research and Treatment of cancer QLQ C30: A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Fayers P. EORTC QLQ-C30 scoring manual, 3rd ed. Brussels: EORTC; 2001.
- Paulson DF. Randomized series of treatment with surgery versus radiation for prostate adenocarcinoma. NCI Mono 1988;(7):127–31.
- Paulson DF, Lin GH, Hinshaw W, Stephani S. Radical surgery versus radiotherapy for adenocarcinoma of the prostate. J Urol 1982;128:502–4.
- Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Hormonal treatment before radical prostatectomy: A 3-year follow-up. J Urol 1998;159: 2013–6; Discussion 2016–7.
- Wahlgren T, Nilsson S, Ryberg M, Lennernäs B, Brandberg Y. Combined curative radiotherapy including HDR brachytherapy and androgen deprivation in localized prostate cancer: A prospective assessment of acute and late treatment toxicity. Acta Oncol 2005;44:633–43.
- Wahlgren T, Brandberg Y, Häggarth L, Hellström M, Nilsson S. Health-related quality of life in men after treatment of localized prostate cancer with external beam radiotherapy combined with (192) ir brachytherapy: A prospective study of 93 cases using the EORTC questionnaires QLQ-C30 and QLQ-PR25. Int J Radiat Oncol Biol Phys 2004;60:51–9.