Abstract
Background. The Swedish brain tumor registry has, since it was launched in 1999, provided significant amounts of data on histopathological diagnoses and on important aspects of surgical and medical management of these patients. The purpose is mainly quality control, but also as a resource for research.
Methods. Three Swedish healthcare regions, constituting 40% of the Swedish population, have had an almost complete registration. The following parameters are registered: diagnosis according to SNOMED/WHO classification, symptoms, performance status, pre- and postoperative radiology, tumor size and localization, extent of surgery and occurrence of postoperative complications, postoperative treatment, such as radiotherapy and/or chemotherapy, other treatments, complications and toxicity, occurrence of reoperation/s, participation in clinical trials, multidisciplinary conferences and availability of a contact nurse.
Results. Surgical radicality has been essentially constant, whereas the use of early (within 72 hours) postoperative CT and MRI has increased, especially for high-grade glioma, which is a reflection of quality of surgery. Survival of patients with high-grade glioma has increased, especially in the age group 60–69. Patients aged 18–39 years had a five-year survival of 40%. Waiting times for the pathological report has been slightly prolonged. Geographical differences do exist for some of the variables.
Conclusion. Population-based registration is valuable for assessment of clinical management, which could have impact on patient care. As a result of short survival and/or the propensity to affect cognitive functions this patient group has considerable difficulties to make their voices heard in society. We therefore believe that a report like the present one can contribute to the spread of knowledge and increase the awareness for this patient group among caregivers and policy makers.
Primary tumors of the central nervous system (CNS) account for approximately 1300 cases or 2.5% of all diagnosed cancers each year in Sweden with a total population of 9.6 million. Although a large number of clinical trials have provided significant amounts of data on how to treat brain tumors, these trials are confined by varying degrees of selection bias and are not necessarily representative of the entire patient population. Neither should the risk for publication bias be underestimated. Population-based registries on primary CNS tumors, which provide systematic and detailed data based on histopathological diagnoses, are currently under development in a number of countries worldwide [Citation1–6]. However, registries that report data not only on CNS tumor subtypes, but also on important aspects of the clinical management of these tumors are much less common. Such registries, including the one described in the present work, might serve as a reference and as a complement to data originating from clinical trials. The objective of this report is to give an overview of the clinical data from a population-based registry with data collected in Sweden between 1999 and 2012 and to suggest the possibilities of using such registries as quality control of clinical management and as a resource for research.
Methods
In 1999 the Swedish Brain Tumor Registry (SBTR) of adult patients with CNS tumors was initiated by the interdisciplinary Swedish National Brain Tumor Trialist Group [Citation7–9].
Swedish health care is organized into six regions that are all responsible for the care and registration of patients diagnosed with tumors in the CNS. Additionally, all regions register their respective CNS tumor patients to SBTR.
In Sweden it is compulsory to report every newly detected cancer case to the National Cancer Registry (NCR). As defined here, the registration rate is the percentage of diagnoses in the SBTR that correspond to diagnoses reported to NCR. High registration compliance is a prerequisite when claiming that a registry is truly population based, and three regions, the Northern, the Uppsala, and the South-eastern regions – which constitute 40% of the Swedish population – have had an almost complete registration (98–100%), with a few exceptions, during the entire period from 1999 to 2012. This high level of coverage is the reason why the present report focuses on these three regions. Over the last few years, the registration compliance has increased for the remaining three regions, and Sweden will soon achieve a registration level allowing for a nationwide comparison of all aspects of management and outcome for patients with primary brain tumors.
Each patient-based dataset is monitored in a web-based system mainly by oncologists, neurologists, and sub-specialized neuro-oncology nurses. All patients aged ≥ 18 years with a diagnosis of a primary CNS tumor are included, with the exception of CNS lymphoma. All statistics based on date of diagnosis refer to the date of the written and signed pathology report.
Since 1999 the following parameters are included: diagnosis according to the Systematized Nomenclature of Medicine (SNOMED)/WHO classification (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369), preoperative symptoms, WHO performance status, which is a five-graded quantification of patients’ general well-being and activities of daily life, pre- and postoperative neuroradiological investigations, tumor size (largest diameter < 4 cm, 4–6 cm and > 6 cm) and localization (left or right, bilateral, central, skull-base, cerebellum or multifocal) [Citation3], extent of surgery (as judged by the surgeon and/or by early postoperative radiology), and any occurrence of possible postoperative complications (infections and hematomas, thromboembolisms, focal neurology or seizures).
The data were analyzed with the statistical software package R version 2.15.2 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria). The publication of the present report was approved by the relevant ethical review board.
Results
Data from the SBTR
For comparison and to put SBTR in an appropriate context a number of figures on incidence and mortality from the Swedish Cancer Registry (SCR) and the Nordic countries (NORDCAN) can be obtained through Supplementary Figures 1–11 (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). Many of the registered parameters are mature enough to be included in this report. Some data has only recently been reported (since 2009), including participation in clinical trials, toxicity, multidisciplinary conferences and availability of a contact nurse and will soon be described separately.
During the period 1999–2012, a total of 5939 diagnoses were registered in the SBTR from the Northern, the Uppsala, and the South-eastern regions. It is noteworthy that there are non-negligible inter-regional differences in the distributions of glioma subtypes (Supplementary Table II, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). As a comparison with regard to the distribution of glioma subtypes, data from the Central Brain Tumor Registry of the United States (CBTRUS) have been included [Citation3]. It should be noted that the data from CBTRUS also include patients aged < 20 years, which constitutes 6.7% of the entire US population. The CBTRUS figures are comparable to those of the SBTR.
There are only small differences in the age distributions of gliomas between the regions from 1999 to 2012, although some variation over time can be seen. Interestingly, the percentage of patients aged ≥ 70 years in the Northern region has increased from 18% to 32% while the corresponding figures for the other two regions show only slight increases.
Performance status is one of the most important prognostic factors and is often a basis for inclusion/exclusion criteria for enrollment into clinical trials. Patients with a WHO performance status 3 and 4 are often excluded because of increased risk for complications. In the SBTR, approximately 10% of the registered patients fall into this category (Supplementary Table III, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369).
Diagnostics
Since patient registration began in 1999, computed tomography (CT) has been the standard radiological procedure for the initial investigation of a suspected brain tumor. Since then, magnetic resonance imaging (MRI) has been used as a complement when a suspicion of a glioma is raised, but there were large inter-regional differences in the use of MRI up to 2007. It was not until the most recent time period that MRI became part of the standard diagnostic procedure ().
Table I. Distribution of patients diagnosed with CT or MRI – high- and low-grade glioma 1999–2012 by region.
Surgery and postoperative radiology
Surgical radicality is an important quality measure for most primary brain tumors. Radical resection in this context is either the surgeons own judgment of per operative radicality or based on radiology, the latter being more sensitive, especially when MRI has been used [Citation10]. The data on high-grade and low-grade gliomas show a slight increase during recent years, while surgical radicality for meningiomas has been essentially constant. A comparison between the regions in terms of extent of resection for high-grade glioma is shown (Supplementary Table IV, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). Postoperative radiology (within 72 hours after surgery) for evaluation of surgical radicality with CT and/or MRI has increased in glioma surgery, especially for high-grade glioma (). In the South-eastern region, resection has been classified as radical twice as frequent as in the other regions (Supplementary Table IV to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). Postoperative MRI has been the routine in the Northern region and the Uppsala region, while the South-eastern region has only recently adopted early postoperative imaging, and this might explain the difference in surgical categorization.
Postoperative mortality
Approximately 5% of the patients succumb to the disease within 30 days after surgical intervention, and this share is consistent between the three regions.
Radiotherapy/oncological treatments
Data for postoperative therapy are still not optimal in the different regions and this makes it difficult to draw firm conclusions about the activity of oncologists and/or radiotherapists in initiating oncological treatment. However, for those hospitals where data have been reported, a clear majority of patients diagnosed with high-grade glioma undergo full-dose radiotherapy (2 Gy fractions up to a total of 60 Gy) with or without concomitant chemotherapy, and roughly one of 10 patients receive other schedules of radiotherapy or medical treatments.
Waiting times
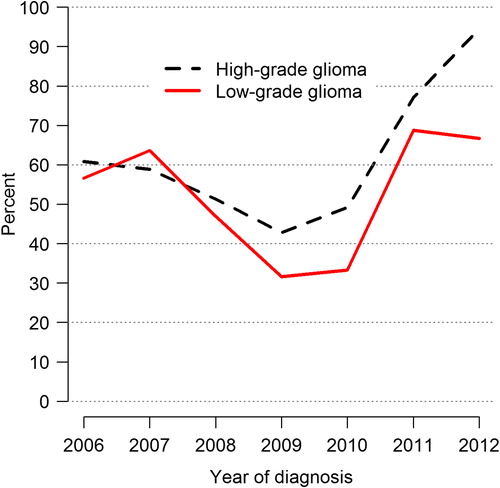
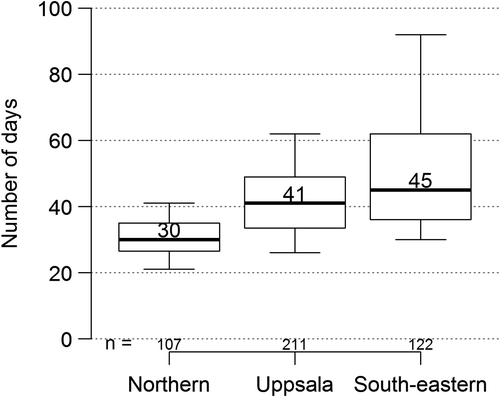
The median time between the radiological finding of a suspected brain tumor and resection has been relatively constant throughout the years at about 18–21 days, but the spread has been considerable. For high-grade gliomas the time between surgery and the pathological report has increased from a median of five days in 1999 to eight days in 2012 with a large spread (Supplementary Figure 12, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). For low-grade gliomas, the corresponding waiting times were six days and 14 days, respectively. The time for start of oncological treatment, most commonly radiotherapy with or without chemotherapy, is added to this waiting time. This issue is more important for high-grade gliomas. In fact, a fraction of the patients with high-grade glioma are waiting longer than the recommended 4–6 weeks [Citation11,Citation12] and some wait for eight weeks or more before initiation of oncological treatment ().
Survival
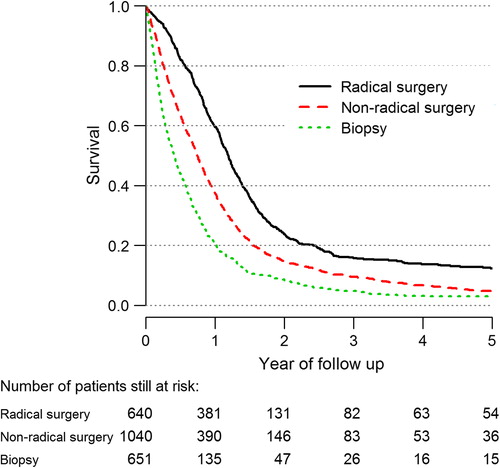
The five-year survival of the different subgroups of glioma from 1999 to 2012 is shown in Supplementary Figure 13 (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). As expected, survival is inversely correlated to tumor grade, and patients with tumors of oligodendroglial origin have a more favorable outcome. The impact of surgical extent is clearly illustrated in in which radical surgery for high-grade glioma is associated with a median survival of 14.4 months (95% CI 13.4–15.1 months). The corresponding figures for partial resections and biopsies are 8.9 months (95% CI 8.4–9.6 months) and 5.0 months (95% CI 4.4–5.6 months), respectively.
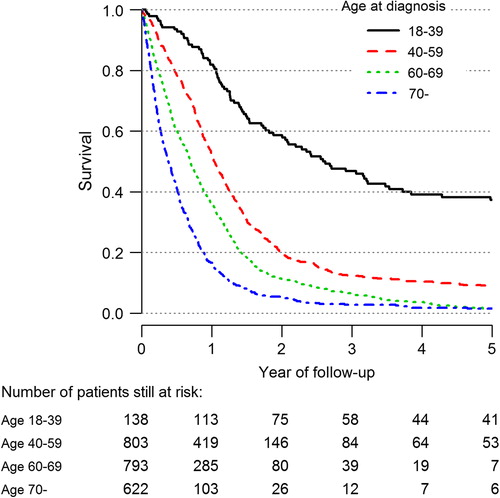
Age is a well-known prognostic factor for high-grade glioma. In the group aged 18–39 years, a five-year survival of 40% was found compared to approximately 10% in the age group of 40–59 years and to only a few percent in those 60 years and older (). All age groups have shown increased median survival since the registry began. The cohort showing the largest improvement in survival is patients aged 60–69 years. Median survival has increased from 5.8 months (95% CI 5.1–7.5 months) during 1999–2003 to 9.8 months (95% CI 8.5–11.7 months) during 2008–2012. The current results are based on updated information compared to our previous report [Citation7].
WHO performance status is also a prognostic factor among high-grade glioma patients where a performance status of 0 or 1 predicts a median survival of approximately one year and a performance status of 2–4 is associated with a median survival of about 4–7 months. Among five-year survivors, therefore, patients with a performance status of 0 or 1 are in the majority (Supplementary Figure 14, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369).
Meningioma
The age distribution of meningiomas (and also gliomas) for all three regions combined is shown in Supplementary Figure 15 (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). The percentage of radical resections exceeds 80% and this figure has been essentially constant over time. A five-year survival of approximately 90% is seen over time.
Discussion
Aims and limitations of the report
This report describes the population-based SBTR, which was launched in 1999. Its primary objective is quality control in the clinic. In the following, a selection of the data will be discussed. The purpose is not to scrutinize or give explanations for each parameter, and such analysis might well be a matter for further research. Rather, our aim is to give an overview of the SBTR and highlight a few relevant issues associated with primary CNS tumor patients and their management in the context of the Swedish healthcare system. Variables, like reoperations, toxicity of postoperative treatment, participation in clinical trials, etc. will soon be presented to a larger extent through an increasing registration compliance of the one-year follow-up form.
There are some limitations to consider when interpreting data from this registry. Changes in results for a specific variable are, as well known, often multifactorial and can be difficult to refine. Numerical changes should therefore mainly be considered as hypothesis generating rather than as issues of causality. Three of six regions (the Northern, the Uppsala and the South-eastern regions), constituting 40% of the Swedish population, have had almost complete registration compliance since the beginning of the registration, and it could be argued that the data are not truly representative of the entire Swedish population for the whole period of 1999–2012. However, as for the remaining regions (the Stockholm, the Western and the Southern regions) the obtained data are comparable with the regions in the present report, regarding, e.g. histopathological diagnoses and distribution of gliomateous subgroups. With these remarks in mind, it should be mentioned that Sweden is a fairly homogeneous country with regard to the healthcare system and to ethnicity. The Stockholm, the Western and the Southern regions are gradually reaching the same registration activity. The main strength with a study based on a population-based registry with near complete registration compliance is that the risk for selection bias is minimized. The need to carry out statistical tests or to calculate confidence intervals to be able to draw inferences on a complete population is also of lessened importance.
Regional differences in glioma subtypes
The differences in glioma subtypes between the included regions are illustrated in Supplementary Table II to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369 (1999–2012). These differences could partly be explained by subjectivity in the judgment of the individual pathologist, but also by a slight shift in the diagnostic criteria during the registration period. Another explanation could be differences in age distribution among diagnosed patients where the most malignant subgroups are more common among elderly patients. The proportion of patients aged ≥ 60 years with high-grade glioma is similar in the three regions. As for the Northern region, it has had a significant increase in the percentage of glioblastoma since the registry started in 1999. Although a real increase in incidence in this specific region cannot be excluded, factors such as inter-regional variations in pathological review have to be further investigated. This is, in fact, an ongoing project among neuropathologists in Sweden that was initiated based on the findings in the registry.
Performance status
There are some inter-regional variations in performance status among patients with high-grade and low-grade glioma (Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369). However, it seems reasonable to assume that there is a certain inter- and intra-observer variation in the judgment of performance status. By experience, the variability in performance status should not exceed 1 point on the five-point scale (0–4). Another reason for these variations could possibly be a difference in neurosurgical activity between regions regarding the most fragile patient group. The percentage of patients with different performance status subject to surgery differs between the regions (Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369), and this could influence treatment activities for the most fragile patient population. For example, the proportion of patients with a performance status of 3 or 4 in the South-eastern region is only half of that in the Northern region. Hopefully, these figures can serve as a basis for discussion among caregivers when selecting patients for different treatment regimens.
Surgery and radiology
Surgery is an important treatment modality for the majority of primary CNS tumors. It also provides the diagnosis that decides the further management of the patient. Therefore, a key issue in a primary CNS tumor registry should highlight aspects of surgery, such as the age distribution of operated patients, radicality, the quality of surgery including the occurrence of postoperative complications. In addition, waiting times between the radiological work up and surgery should also be included. However, there are a number of caveats concerning the validity of aspects of surgical quality when comparing the different regions and time periods. Radicality can be defined macroscopically and/or by postoperative imaging. By experience, radiological radicality is often more sensitive than the peroperative judgment, and postoperative MRI (within 72 hours) is more sensitive than postoperative CT. The increased use of MRI is encouraging because of its higher sensitivity for brain tumor diagnostics [Citation10]. Postoperative MRI has been the routine in the Northern region and the Uppsala region, while the South-eastern region has only recently adopted early postoperative imaging, and this might explain the difference in surgical categorization.
A somewhat unexpected decrease in the use of postoperative radiology, especially of low-grade glioma, during the 2008–2010 period is evident ().The reason for this is presently investigated.
An observation is that radical surgery seems to correlate with an increased survival among high-grade glioma patients (). The difference in survival between patients undergoing ‘radical’ surgery in comparison with patients treated with partial resection is not easy to explain by selection bias only. These two groups are probably of a comparable performance status because surgical resections are in most cases intended to be as radical as safely possible. The proportion of radical resections versus partial resections have been of the same magnitude among patients aged 18–64 years, but surgeons operating on patients aged ≥ 65 years are more prone to perform a biopsy.
It should be emphasized that postoperative radiology, i.e. CT or MRI within 72 hours after surgery, is not only a quality control of the surgical intervention [Citation13] but can also serve as a baseline evaluation for postoperative therapy and as guidance for target definition for radiotherapy. In addition, the possibility that a postoperative examination can reveal residual tumors, which might be the target of complementary surgery, should be considered. Thus, there are several reasons to perform an early postoperative examination, and this should probably be with MRI because of its higher diagnostic sensitivity.
Postoperative complications and mortality
Postoperative complications are either directly related to surgery, e.g. postoperative hematomas and/or ischemia and wound infections, or are indirectly associated, including complications, such as thrombosis and infections from other origins. Although the mortality rate of 5% within 30 days after tumor surgery should be interpreted with caution, it is important in the quality control of the care of brain tumor patients. Complications could possibly be minimized by the development of surgical techniques and educational measures.
Radiotherapy and oncological treatments
Even though the registration compliance has not been complete regarding follow up (range 64–97% for the years 2009–2012), it is reasonable to conclude that a clear majority of high-grade glioma patients receive postoperative oncological treatment with radiotherapy and/or chemotherapy. Here is included a fraction of patients, roughly 10%, who receive other treatment regimens, such as hypofractionated radiotherapy or single agent chemotherapy.
Waiting times
An important objective in patient care is to have short waiting times for surgery.
There are reasons for concern when studying this for high-grade gliomas. These tumors are among the most rapidly growing neoplasms, and this emphasizes the necessity for measures to minimize these times. Nevertheless, the waiting time between the radiological diagnosis and surgery for high-grade glioma has been fairly constant (presently almost 3 weeks). Another quality aspect is the waiting time between resection and the pathological report, which is the foundation for the choice of postoperative management. This time has increased somewhat (Supplementary Figure 12 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369) (presently approximately 1 week). The psychosocial aspect of the individual patient having to wait several weeks for diagnosis is of importance. There is no strict consensus as to the recommended time interval between surgery and the start of radiotherapy and/or chemotherapy. Waiting times between two and six weeks are in accordance with opinions from the literature, and there is no evidence justifying a longer waiting time [Citation11,Citation12]. Even here it is noteworthy that one of the regions exceeds this time, and some patients have to wait even more than 60 days (). A possible explanation for these variations can be contraindications for earlier treatment start, but other reasons are presently being investigated.
Survival
Survival has increased since the start of the registry in 1999 for all age groups diagnosed with a high-grade glioma, and especially among patients aged 60–69 years. One reason could be an increase in surgical radicality. However, this issue is not confirmed by registry data. A more plausible explanation is the increased use of early postoperative imaging in recent years (). This means that a fraction of cases that might have previously been classified peroperatively as radical could today, because of more sensitive postoperative imaging techniques, be classified instead as partial resections. An increased active attitude among neurosurgeons is supported by improvements in microsurgical techniques [Citation13,Citation14]. As for meningioma the slight decrease of survival is probably due to stage migration, i.e. a shift in surgical indication towards more advanced cases. Of note, the meningioma cases in this report consist of a selected population of operated meningiomas. In other words, this only includes cases where a morphological diagnosis was available, and patients with a diagnosis based on radiology alone are not included. A more active surgical approach could possibly imply that patients operated even with subtotal resections might benefit from treatment. Obviously, the higher risk for complications should be considered.
Another more accepted explanation for improved survival among high-grade glioma patients is the use of concomitant radiochemotherapy, which was introduced in 2005 [Citation15].
PROM and PREM
Much effort is presently being put into the development of patient-reported outcome measures (PROM) and patient-reported experience measures (PREM). A spread opinion is that these are probably more sensitive than conventional methods in assessing improvements in medical care and will likely contribute significantly to the evaluation of the effects of new treatment strategies and new surgical approaches. They could also act as instruments to evaluate changes in other quality measures, such as waiting times, rehabilitation, and palliative care. These parameters are now underway to be included in the SBTR.
International collaboration
International collaboration within the medical profession in order to reach consensus in diagnosing and managing patients is of utmost importance, and this can be facilitated by the development of population-based registries worldwide. Such registries are useful resources for researchers, neuropathologists, clinicians, and patient families to achieve a higher implementation of quality measures in the daily care of patients suffering from CNS tumors [Citation3].
Conclusion
Primary brain tumors, especially high-grade gliomas, but also in many respects other CNS tumors, are among the most devastating diseases in oncology. As a result of rapid disease progression that results in short survival times and/or reduced cognitive functions [Citation16], this patient group has considerable difficulties in making their voices heard in society. We believe that a registry like the present one, apart from being a basis for future improvements in patient care, can also contribute to the dissemination of knowledge and increase the awareness for this patient group in society and among policy makers.
Supplementary material available online
Supplementary Figure 1–15 and Table I–IV to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.957369
ionc_a_975369_sm9198.pdf
Download PDF (1.2 MB)Declaration of interest: RH, MB and TA are members of the Swedish National Brain Tumor Trialist Group and clinical investigators in collaboration with Roche.
References
- Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, et al. The Austrian Brain Tumor Registry: A cooperative way to establish a population-based brain tumour registry. J Neurooncol 2009;95:401–11.
- Baldi I, Gruber A, Alioum A, Berteaud E, Lebailly P, Huchet A, et al. Descriptive epidemiology of CNS tumors in France: Results from the Gironde Registry for the period 2000–2007. Neuro Oncol 2011;13:1370–8.
- Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 2012;14(Suppl 5):v1–49.
- Kaneko S, Nomura K, Yoshimura T, Yamaguchi N. Trend of brain tumor incidence by histological subtypes in Japan: estimation from the Brain Tumor Registry of Japan, 1973–1993. J Neurooncol 2002;60:61–9.
- Ronning PA, Helseth E, Meling TR, Johannesen TB. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol 2012;14:1178–84.
- Sehmer EA, Hall GJ, Greenberg DC, O’Hara C, Wallingford SC, Wright KA, et al. Incidence of glioma in a northwestern region of England, 2006–2010. Neuro Oncol 2014;16:971–4.
- Asklund T, Malmström A, Björ O, Blomquist E, Henriksson R. Considerable improvement in survival for patients aged 60–84 years with high-grade malignant gliomas – data from the Swedish Brain Tumour Population-based Registry. Acta Oncol 2013;52:1041–3.
- Asklund T, Björ O, Malmström A, Blomquist E, Henriksson R.[Survival in malignant gliomas has increased the last decade. Analysis of quality data]. [Article in Swedish]. Läkartidningen 2012;109:875–8.
- Bergenheim T, Malmström A, Bolander H, Michanek A, Stragliotto G, Damber L, et al. [Registration on regional basis of patients with primary brain tumors. Regional differences disclosed]. [Article in Swedish]. Läkartidningen 2007;104:332–8, 340–1.
- Clarke JL, Chang SM. Neuroimaging: Diagnosis and response assessment in glioblastoma. Cancer J 2012;18:26–31.
- Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol 2007;85:339–43.
- Lawrence YR, Blumenthal DT, Matceyevsky D, Kanner AA, Bokstein F, Corn BW. Delayed initiation of radiotherapy for glioblastoma: How important is it to push to the front (or the back) of the line? J Neurooncol 2011;105:1–7.
- Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, et al. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J Neurosurg 2012;117:851–9.
- Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2014;115:3–8.
- Weller M, Bent M, Hopkins K, Tonn JC, Stupp R, Henriksson R, et al for the European Association for Neuro-Oncology (EANO) Task Force on Malignant Glioma. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncology 2014;15: e395–403.
- Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastomamultiforme: A review. J Neurooncol 2011;104: 639–46.