Many pediatric cancer patients require cranio-spinal irradiation (CSI) as part of curative treatment for various childhood central nervous system malignancies. Notably, medulloblastoma represents the most common malignant pediatric brain tumor requiring CSI. Aggressive treatment regimens in this setting result in relatively high cure rates, with five-year overall survival (OS) ranging from 72% to 85% [Citation1]. As a result of long-term survival following comprehensive treatment, many patients are at risk for the development of late effects [Citation2].
Standard photon beam irradiation of the cranio-spinal axis has been associated with late effects to include vertebral growth arrest, cardiac dysfunction, restrictive lung disease and gonadal dysfunction [Citation3]. Furthermore, pediatric patients receiving central nervous system (CNS) irradiation may be subject to a number of well-characterized treatment-related late effects to include neurocognitive deficits, endocrine dysfunction, secondary malignancy and hearing deficits [Citation4]. The capacity for increased target conformality of proton beam therapy (PBT) has been proposed to reduce late effects, with ongoing investigation into clinical outcomes [Citation5,Citation6].
Accumulating evidence from the literature demonstrates a linkage between the development of diabetes mellitus (DM) and total body/abdominal irradiation [Citation7,Citation8]. A recent multi-institution study, by deVathaire et al. demonstrated a dose-response relationship between radiation dose to the pancreatic tail and risk of subsequent DM in childhood cancer survivors [Citation9]. deVathair et al. reported on the long-term follow-up of pediatric patients treated with radiation, involving dose to the pancreas. An 11.5-fold relative increased risk of DM was noted with doses greater than 10 Gy to the pancreas, specifically the tail of the pancreas. Another recent study by van Nimwegen et al. contributed further support to a relationship between pancreatic and pancreatic tail radiation and the increased risk of developing DM [Citation10]. Here the authors demonstrated that a mean dose to the pancreatic tail of ≥ 36 Gy resulted in a 2.58-fold increased risk of developing DM [Citation10]. Collectively these data suggest the potential radiosensitivity of pancreatic islet cells responsible for insulin secretion [Citation11]. These findings support identifying the pancreas as a radiosensitive organ at risk (OAR) during CSI, which has not previously been standard practice.
To better assess pancreatic dosimetry using conventional photon techniques and the differential capacity for pancreatic sparing with techniques of increased conformality, this study compares CSI approaches using conventional three-dimensional conformal photon therapy (3DCRT), inverse-planned intensity-modulated radiotherapy using helical tomotherapy (HT), and PBT.
Material and methods
Five pediatric patients with average-risk medulloblastoma consecutively enrolled on an IRB-approved prospective registry were selected. All patients received CSI to 23.4 cobalt gray equivalents (CGE) using PBT with concurrent weekly vincristine chemotherapy. The original datasets were acquired using a General Electric LightSpeed 16 slice scanner at 1.2 mm slice increments. Computed tomography (CT) data sets were reconstructed to larger slice spacing for importation. MRI fusions were performed in Velocity TM (Palo Alto, CA, USA) planning system for target volume delineation. According to institutional PBT protocol, planning target volume (PTV) included the entire vertebral bodies except for the anterior 0.3cm. CT and structure data sets were imported into XiO treatment planning software (Stockholm, Sweden), where PBT plans were generated per institutional protocol. Straight PA fields were used to treat the vertebral bodies. Planning was carried out utilizing uniform scanning with apertures to conform the lateral edges and compensators to shape the distal end of the beam. The proximal and distal ends of the beam were optimized to cover the PTV with the 95% isodose line.
Initial PBT plans did not include the pancreas or pancreatic tail as OARs. These structures were delineated on the original CT data sets, and cases were re-planned using conventional 3DCRT and HT. The 3DCRT cranio-spinal plans were also performed using XiO treatment planning software and used opposed lateral cranial fields junctioned to posterior spinal fields with 6 MV photons. Guidelines for coverage followed the PBT protocol of 95% coverage. Multiple HT plans were also developed for each patient. HT plans delivering the lowest dose to the whole pancreas and pancreatic tail without compromising 95% target coverage were selected. Plan assessment and selection included evaluation of dose to all standard OARs. Low dose to other OARs was not sacrificed at the expense of pancreatic dose optimization. Weight point locations of the beams were modified to obtain distal coverage. Lateral conformality was obtained using a multileaf collimator. Optimization was carried out to obtain full coverage to the PTV with the 95% isodose line.
Endpoints included mean dose (Dmean), maximum point dose (Dmax), and volumes receiving 5 Gy, 10 Gy and 20 Gy (V5,V10 and V20, respectively). Comparisons were made using analysis of variance (ANOVA); statistical significance was assigned to p-values < 0.05.
Results
Target goals were met in all cases such that all three modalities covered the PTV with the 95% isodose line. Pancreatic dose volume results are displayed in .
Table I. Comparison of dose metrics as a function of modality. T-test and analysis of variance (ANOVA); statistical significance was assigned to p-values < 0.05.
Mean dose deposited to whole pancreas and pancreatic tail was significantly greater with 3DCRT compared to HT or PBT (p < 0.001) (). 3DCRT also resulted in greater maximum dose values delivered to whole pancreas and pancreatic tail when compared to HT or PBT (p = 0.015, p < 0.001). The maximum dose values for whole pancreas were similar between HT and PBT (p = 0.99). V5 of whole pancreas was higher with 3DCRT than either HT or PBT, but V5 of pancreatic tail was similar between 3DCRT and HT. V10 was significantly lower with HT and PBT when compared to 3DCRT for both whole pancreas and pancreatic tail (p < 0.001). Similar differences were observed when comparing V20 for whole pancreas and pancreatic tail. V20 was also higher with 3DCRT compared to HT or PBT (p < 0.001). Representative color-wash dose distributions are shown for 3DCRT, PBT and HT in a single representative patient (). V10 values for HT and PBT were similar with regard to pancreatic tail in our study (p = 0.36). Proton beam treatment plans did not result in significant reduction in V20 when compared to HT in our analysis for either whole pancreas or pancreatic tail (p = 0.36) ().
Figure 1. Dose-volume histograms. (A) Whole pancreas comparison of proton therapy, helical tomotherapy and 3DCRT. (B) Pancreatic tail comparison of proton therapy, helical tomotherapy and 3DCRT.
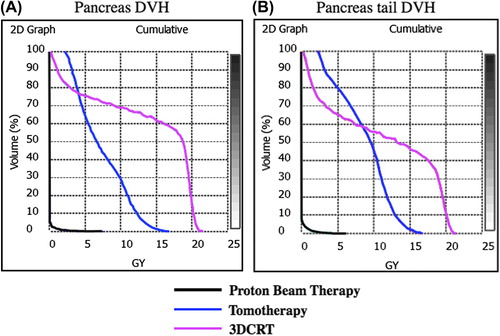
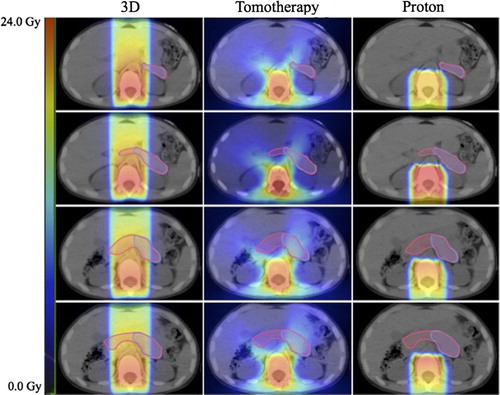
Figure 2. Dosimetric comparison of modalities: cranio-spinal irradiation for medulloblastoma. Left to right: 3D conformal radiotherapy, tomotherapy, proton therapy. Contoured structures: pancreas (pink), pancreatic tail (violet). Scale bar: 0–24 Gy.
While Dmean values to whole pancreas and pancreatic tail were lower with HT compared to 3DCRT, PBT resulted in further reductions in Dmean when compared to HT (, Supplementary Figure 1A and B, to be found at online http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.978368). Dmax for whole pancreas and pancreatic tail was highest with 3DCRT, but PBT did not provide any improvements relative to HT (Supplementary Figure 1A and B to be found at online http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.978368). PBT plans also did not result in significantly lower V5 values for whole pancreas and pancreatic tail when compared to HT. V10 of whole pancreas was lower with PBT when compared to HT.
Discussion
Teinturier et al. first reported that 6.6% of survivors of nephroblastoma treated with abdominal radiation developed diabetes in early adulthood [Citation12]. Shortly thereafter Cicognani et al. sought to determine a cellular mechanism and tested beta cell dysfunction as a result of irradiation in the setting of nephroblastoma treatment [Citation13]. Here they demonstrated that 31.8% of those individuals treated with abdominal radiation mounted an inadequate first-phase of the insulin response following intravenous glucose challenge in comparison to only 4.5% of non-irradiated controls [Citation13]. Subsequently, the childhood cancer survivor study (CCSS) reported a 1.8-fold risk of the development of diabetes in patients treated with radiation for childhood malignancies, 7.2-fold greater after total body irradiation and 2.7-fold greater following abdominal irradiation [Citation7,Citation8].
Rodent and human models suggest that the overall majority of islet cells reside within the pancreatic tail [Citation11,Citation14]. Collectively, however, the head, body and uncinate contain a greater total number of beta cell islets than tail alone, suggesting a more diffuse distribution of beta cell total mass [Citation15,Citation16]. Wittingen et al. demonstrated that while the greatest proportion of islets resided within the tail, total islet numbers were greater when summating head, body and uncinate process [Citation11]. Indeed it is evident that the majority of islets are located within the tail, however, head, body and uncinate contain a greater total number of islet cells [Citation11,Citation16]. As a result of beta cell radiosensitivity and an anatomically demonstrated diffuse distribution of beta cells, we believe it is clinically important to investigate and implement techniques of radiation delivery that could reduce dose to the entire pancreas.
PBT and HT were herein shown to reduce non-target mean and maximum dose to the whole pancreas and pancreatic tail in comparison to 3DCRT. Clinically these differences are likely of significance as greater mean doses, specifically > 10 Gy have been shown to predict for up to an 11.5-fold increased risk of the development of DM. While both PBT and HT resulted in greater non-target pancreatic sparing, PBT resulted in reduced mean dose deposition to whole pancreas and pancreatic tail compared to HT and 3DCRT. The volume of whole pancreas receiving 10 Gy was also decreased with PBT (Supplementary Figure 1A to be found at online http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.978368). Taken collectively and in accordance with data from the literature, these dosimetric benefits have the potential to result in a relative reduction in the development of DM in the setting of CSI for medulloblastoma.
The current study provides dosimetric data demonstrating the utility of PBT and HT in reducing total dose to pancreas and pancreatic tail in the setting of CSI for medulloblastoma. While data from recent studies have correlated the risk of developing DM with pancreatic dose, these dosimetric data may be leveraged to inform clinicians of the potentially underappreciated risk of DM in pediatric patients treated with CSI. These data in conjunction with previously reported outcome data highlight the possibility that traditional techniques of photon delivery in the setting of CSI for medulloblastoma may contribute to the development of DM in long-term survivors and that non-target pancreatic dose may be reduced with techniques of increased conformality, such as PBT and HT. Such pancreatic dose attenuation may ultimately result in the reduced development of DM among survivors of medulloblastoma.
While limited by the inclusion of small patient numbers and dosimetric nature of the study, these data provide quantifiable evidence of the utility of technologies with increased conformality, specifically with regard to non-target dose to the pancreas. While data by de Vathaire and Nimwegen et al., may demonstrate different dose response relationships with regard to subsequent development of DM, collectively these data do support the importance of pancreatic sparring in long-term survivors. The authors are also aware of data which suggests that the development of DM in medulloblastoma patients may be related to metabolic syndrome which was not addressed here and not within the scope of this article.
Sufficient previously reported data in conjunction with results reported herein may be utilized to inform clinicians of an underappreciated late effect of abdominal radiation. Further, these data may be leveraged to direct cranio-spinal radiation in the setting of medulloblastoma and to provide support for the utilization of modalities of increased conformality to reduce the likelihood of DM development in long-term survivors.
Supplementary material available online
Supplementary Figure 1 to be found at online http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.978368.
ionc_a_978368_sm2262.pdf
Download PDF (136.6 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer 2012;118:1313–22.
- von Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer 2009;45:1209–17.
- Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/ primitive neuro-ectodermal tumors: Spinal theca irradiation. Int J Radiat Oncol Biol Phys 1997;38:805–11.
- Morris EB, Gajjar A, Okuma JO, Yasui Y, Wallace D, Kun LE, et al. Survival and late mortality in long-term survivors of pediatric CNS tumors. J Clin Oncol 2007;25:1532–8.
- Yock TI, Caruso PA. Risk of second cancers after photon and proton radiotherapy: A review of the data. Health Phys 2012; 103:577–85.
- Merchant TE. Proton beam therapy in pediatric oncology. Cancer J 2009;15:298–305.
- Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 2008;100:1368–79.
- Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the childhood cancer survivor study. Arch Intern Med 2009;169:1381–8.
- de Vathaire F, El-Fayech C, Ben Ayed FF, Haddy N, Guibout C, Winter D, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: A retrospective cohort study. Lancet Oncol 2012;13: 1002–10.
- van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Raemaekers JM, Kremer LC, et al. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma. J Clin Oncol 2014;32:3257–63.
- Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg 1974; 179:412–4.
- Teinturier C, Tournade MF, Caillat-Zucman S, Boitard C, Amoura Z, Bougneres PF, et al. Diabetes mellitus after abdominal radiation therapy. Lancet 1995;346: 633–4.
- Cicognani A, Cacciari E, Mancini AF, Pasini A, Salardi S, Salmi S, et al. Abnormal insulin response to glucose following treatment for Wilms’ tumor in childhood. Eur J Pediatr 1997;156:371–5.
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci 2006;103:2334–9.
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010;59:1202–10.
- Kilimnik G, Kim A, Jo J, Miller K, Hara M. Quantification of pancreatic islet distribution in situ in mice. Am J Physiol Endocrinol Metab 2009;297:E1331–8.
