Abstract
Background. The human epidermal growth factor receptor complex (EGFR-1, HER2, HER3 and HER4) plays an important role in pathogenesis of solid tumours. We have previously reported high expression of HER3 in 70% of primary colorectal cancer (CRC) and that high expression were linked to a worse clinical outcome. The purpose of this study is to evaluate the HER3 expression in primary CRC and metastases.
Material and methods. Tissue samples from primary CRC, corresponding lymph node metastases and liver metastases from 107 patients were analysed by immunohistochemistry.
Results. Of 107 patients, 80% showed high HER3 expression in primary CRC tumours and 81% of the stage III patients presented high expression in the lymph node metastases. All patients had liver metastases and 82% presented high HER3 expression. HER3 expression in primary tumour correlated with expression in the corresponding lymph node metastases (r = 0.65, p < 0.001) and in the liver metastases (r = 0.45, p < 0.001). A correlation between HER3 expression in corresponding lymph node and liver metastases (r = 0.65, p < 0.001) was seen.
Conclusion. High HER3 expression is seen in about 80% of primary CRC, corresponding lymph node metastases and liver metastases. There is a correlation between HER3 expression in primary tumour and metastases in CRC.
Colorectal cancer (CRC) is the third most common cancer in the world [Citation1]. Among CRC patients, 15–20% have liver metastases at diagnosis, another 20% develop metastases later in the course of disease [Citation2,Citation3]. CRC spread to the liver is potentially curable with surgery in 20% of the patients today. Liver surgery for metastases has markedly increased survival. In population-based studies, a five-year survival rate of as high as 45–50% have been reported in the group of patients where liver resection for metastases of CRC was possible [Citation4,Citation5].
The tumour stage (I–IV) based on the TNM (tumour, node, metastases) classification is still the strongest and most widely used prognostic factor in CRC [Citation6]. Improved chemotherapy and surgical techniques in the last decades have increased survival for patients with metastatic CRC [Citation4]. To further improve outcome in CRC, it is crucial to identify specific targets for anticancer therapy and to find specific and sensitive predictive and prognostic biomarkers [Citation7].
HER3
The human epidermal growth factor receptor complex (EGFR-1, HER2, HER3 and HER4) consists of cell surface tyrosine kinases (RTK's) that play important roles in pathogenesis of solid tumours [Citation8–11]. RTK's activate intracellular signalling in response to extracellular ligand binding. Neuregulines are known ligands for HER3. Receptors can interact and form homodimers or heterodimers. HER2, the most common dimer partner, is not ligand regulated and is known to be non-stop active. The HER3 receptor lacks intrinsic tyrosine kinase activity, which makes heterodimerisation with other HER members essential to initiate signal transduction. Phosphorylation of the intracellular domain of HER3 is carried out by its dimerisation partner. The dimerisation of HER3 and other HER members directly activates the PI3K pathway and therefore HER3 is a possible target for specific therapeutic agents [Citation12,Citation13]. Inappropriate signalling can occur as a result of receptor over expression leading to cell proliferation, resistance to apoptosis, cancer cell motility, angiogenesis and formation of metastases. Interdependency and complementary functions between different members of the HER complex are common [Citation14–16]. The most active heterodimer is the HER2/HER3 dimer and is of particular interest with a central role in the PI3K/AKT and the MAPK pathways which are activated in cancer [Citation13,Citation17–18]. High expression of HER3 is an established negative prognostic indicator in some malignancies, i.e. breast, gastric and ovarian cancer [Citation11]. It is also reported that high HER3 expression is associated with decreased survival in CRC [Citation11,Citation19]. Few studies are performed on HER3 expression in primary CRC and the correlation to HER3 expression in corresponding metastases and results tend to diverge [Citation11,Citation19–21]. In a previous study including 236 CRC patients we have reported a correlation between HER3 expression in primary tumour and lymph node metastases and that a high HER3 expression was associated with shorter OS and disease-free survival (DFS) [Citation19]. The aim of this study was to evaluate the correlation between HER3 expression in primary CRC, lymph node and liver metastases. If correlation exists and there is a high percentage of HER3 expression, there is a therapeutic possibility for HER3 blocking agents also in the metastases.
Material and methods
Patients
The patients in this study was derived from a population-based cohort (n = 255) undergoing liver resection for CRC metastases between 2004 and 2009 at the Unit of Hepatobiliary Surgery, Karolinska University Hospital. The primary CRC tumours were resected between 1999 and 2009 in different Swedish Hospitals. Paraffin embedded blocks from primary CRC tumours (n = 107), corresponding nodal metastases (n = 62 patients had metastatic nodes) and liver metastases (n = 107) were collected. Data from patient's medical records regarding age, gender, tumour location, TNM stage, number of lymph nodes sampled at primary surgery, neoadjuvant treatment, synchronous or metachronous metastases, dates for surgical procedures and survival were retrieved. Information on tumour histopathology was derived from pathology reports ().
Table I. Patients and primary CRC tumour characteristics and HER3 expression in primary tumour.
Liver metastases that appeared less than 6 months after the removal of the primary tumour were considered as synchronous. Liver metastases that appeared more than 6 months after the removal of primary tumour were considered as metachronous. Survival time was calculated in months after the primary CRC surgery. The Board of Research Ethics at Karolinska University Hospital has approved the study.
Immunohistochemistry
Tumour specimens were derived from formalin-fixated, paraffin-embedded, CRC tumours, sliced in 4-μm thick sections. Sections were deparaffinised in xylene, rehydrated in ethanol and washed in distilled water. To quench endogenous peroxidation, 3% hydroperoxidase in tap water was added. To reduce background staining, incubation in 10% goat serum for 30 minutes was performed. A HER3 monoclonal rabbit antibody (Abcam, SP71 ErbB, ab 93739, dil 1:800) was added and left at + 4°C overnight. The samples were incubated with an amplification system, EnVision™ (DakoCytomation) for 30 minutes. Visualisation of staining with 3,3’-diaminobenzidine tetrahydrochloride (DAB, DakoCytomanion) was carried out followed by Mayers Haematoxylin.
Scoring system
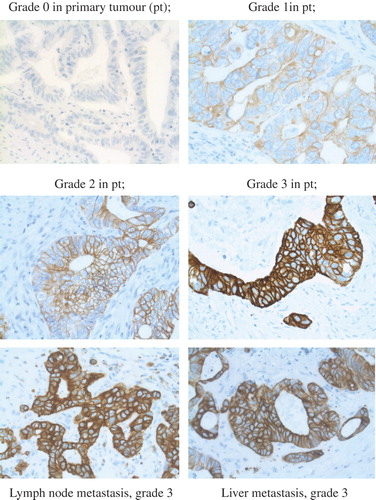
Since there is yet no consensus for the IHC HER3 staining procedure in CRC, the Hercept test, DAKO interpretation manual for gastric cancer, was modified and followed as guidance. Slides from the primary tumour, lymph node metastases and liver metastases from the first liver resection were evaluated using a light microscope by two independent observers (F.L. and M.H.). Disagreements (< 5%) were reviewed followed by conclusive judgment. In most cases, two slides from each tumour were available for analysis. Cytoplasmic or background staining was not graded. The lower limit of positive expression of HER3 was set at 10% stained CRC cells according to literature [Citation10,Citation20,Citation21]. The entire slide of the tumour or metastasis was evaluated. An approximation was done on the percentage of stained cancer cells. The intensity of membrane staining was graded 0–3, where grades 0–1 were categorised as low expression and grades 2–3 as high expression of membranous HER3 (). Staining pattern was considered when scoring according to the Hercept test. Absent stain or occurrence in < 10% was categorised as negative, grade 0. Very faint membrane staining, only present in part of the membrane, was considered grade 1. Weak to moderate stain that was complete or basolateral was considered grade 2 and strong stain that was complete or basolateral was defined as grade 3.
Statistics
χ²-test was performed to examine relationships between patient's demographics, tumour characteristics and HER3 expression. Survival analysis was performed using the Kaplan-Meier method. Correlation analysis was performed with the Spearman's Ranks test. Cox regression was used in the univariate analysis. The results were considered significant if p < 0.05. Calculations were performed using Statistica version 10 (StatSoft, Tulsa, OK, USA).
Results
Patients and clinicopathological characteristics
The median age of the patients was 63 years, 60% were males and 40% were females. Liver metastases in 61/107 (57%) of the patients were synchronous. The median number of analysed lymph nodes (metastatic or benign) was 13 and 63% of the patients had 12 or more lymph nodes analysed (). A majority of the patients, 62/107 (58%) had metastatic lymph nodes at the time of primary surgery. The median time between primary surgery until operation of patients with metachronous liver metastases was 24 months (range 8–68 months). The median follow-up time for all living patients was 74 months.
HER3 expression in primary tumour related to clinicopathological parameters
Expression levels of HER3 in the primary tumour did not correlate to gender, age, stage, localisation of the tumour (colon/rectum), differentiation grade or preoperative therapy.
Expression and correlation of HER3 in primary tumour, lymph node metastases and liver metastases
Eighty-six patients (80%) showed high HER3 expression in primary CRC tumours and 50/62 (81%) had high expression in the lymph node metastases. All 107 patients had liver metastases and of them, 88 (82%) had high HER3 expression in the liver metastases. No difference was seen between the synchronous and metachronous groups regarding HER3 expression in the primary tumour or in the liver metastases. HER3 expression in primary tumour correlated with expression of HER3 in the liver metastases (n = 107) (r = 0.45, p < 0.001) and in the lymph node metastases (n = 62) (r = 0.65, p < 0.001). A correlation was also seen between HER3 expression in liver and lymph node metastases (n = 62) (r = 0.65, p < 0.001).
HER3 expression and overall survival (OS)
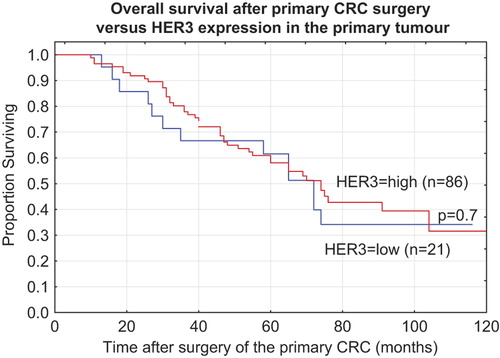
The median survival time for all patients from the primary surgery was 60 months (range 10–168 months). Survival data according to high and low HER3 expression was calculated in months after the primary CRC surgery (). There was no correlation between HER3 expression and OS neither using primary surgery nor using liver resection as a reference point. HER3 expression in the primary CRC tumour was not prognostic for the clinical outcome in the subgroup of patients treated with surgery alone or in the different subgroups treated with radiotherapy, radiochemotherapy or chemotherapy.
Discussion
HER3 is a member of the EGFR complex that consists of an interacting network of receptors and ligands that must be considered as a unity. HER3 has potential as a biomarker and its expression in primary CRC, expression in corresponding metastases and the relationship to clinical outcome are therefore interesting. In the present study, we have shown that expression of HER3 in CRC remains high (about 80%) during progression of cancer disease from primary tumour to lymph node metastases and to liver metastases in the same individuals and that there is a correlation between HER3 expression in tumour and metastases. The relationship between HER3 expression in tumour and liver metastasis showed intermediate strength (r = 0.45) in correlation and a bit stronger association was seen between primary tumour and lymph node metastasis (r = 0.65).
The study raises some questions that are appointed as follows. The number of patients in this study is 107 and derived from a population-based cohort of 255 patients undergoing CRC metastatic liver surgery. The intention was to collect all primary tumours from in total 12 different hospitals, logistic problems when collecting made the number 107. A comparison was made of the 107 patients to the entire cohort and survival was the same, indicating no severe skewness. The time span when primary tumours were resected was between 1999 and 2009. Over time, surgical techniques have changed, diagnostic tools have improved and intention to follow regional and national guidelines has increased. Immunohistochemistry was used to detect HER3 and is widely used in clinical praxis. However, it is a semi quantitative method to detect proteins and is to some extent dependent on the investigators. Validation of our results using another quantitatively method, Western blot or mass spectrometry, is recommended in future studies. The discrepancies in expression of HER3 in CRC observed in different studies can be related to multiple causes, i.e. different staining protocols, antibodies, fixation time and variation in scoring methods (Rajkumar score, Hercept score, other). There is a risk of under staging when less than 12 nodes are analysed [Citation22]. In this study a median number of 13 lymph nodes were analysed in the primary tumour specimen. In total 63% of the patients had 12 or more lymph nodes analysed. To our knowledge this is the largest study (n = 107) analysing HER3 expression in primary CRC and corresponding lymph node and liver metastases.
This study shows a correlation between HER3 expression in primary CRC and lymph node metastases. The same correlation was seen in our former study including 236 CRC patients and corresponding lymph node metastases [Citation19]. In our earlier study, HER3 expression in primary CRC was of prognostic value and a high expression was associated with worse clinical outcome but not in our current study. This could be explained by that the study populations examined differ in one important aspect; in this study all patients have metastatic disease which was not the case in the previous study. This may imply that we now examined a selected group that represents a more biologically aggressive disease [Citation23]. When separately analysing the subgroups of patients with synchronous versus metachronous CRC liver metastases regarding HER3 expression in the primary tumour and survival, we could not find any difference although that could have been expected. This may be due to the relatively small sample size or to actual lack of biological difference. In a study by Ljuslinder et al., 67% of primary CRC tumours (n = 64) showed high HER3 expression and there was a correlation with expression of HER3 in corresponding lymph node metastases, both membrane and cytoplasmic staining were considered [Citation21]. In a study by Wei et al. including 49 patients, they reported a HER3 expression in 16% of the primary CRC and 18% in corresponding lymph node metastases. They did not find membrane staining of HER3 expression but cytoplasmic. HER3 expression did correlate between tumour and lymph node metastases [Citation20]. Only nine patients had liver metastases analysed and two expressed strong HER3. The two above mentioned studies had a small number of included patients and may cause uncertainty in the correlations. In a meta-analysis, Ocana et al. evaluates the prognostic significance of HER3 expression in different solid tumours [Citation11]. HER3 was appointed as a robust prognostic marker with a correlation to worse survival in CRC. Ocana also found that there was no difference in survival in studies analysing cytoplasmic or membranous staining.
Our study population consisted of CRC patients with synchronous and metachronous liver metastases. Slesser et al. investigated the tumour biology of colorectal liver metastases, especially the aspect of molecular marker expression. They concluded that most genetic aberrations in primary tumours were maintained in the CRC liver metastases [Citation23]. In another review regarding CRC liver metastases by Tan et al., the same conclusion was drawn. Tan stated that synchronicity in liver metastases suggests a more aggressive disease but whether there are biological differences between the primaries of the synchronous and the metachronous groups remains undetermined [Citation24]. When comparing our finding of maintained HER3 status in CRC metastases analogously to Miglio et al., concerning KRAS mutational status, which is maintained in corresponding CRC metastases [Citation25], one can suppose that there is an underlying oncologic advantage of this phenomena regarding HER3 and KRAS.
In the general metastatic CRC setting, one study showed that patients (n = 84) with high HER3 expression in the primary tumours, treated with EGFR inhibiting antibody therapy (cetuximab), had a worse outcome compared to patients with a low HER3 expressing tumour. This implicates that HER3 might interact with the response to anti-EGFR therapy in metastatic CRC [Citation26].
Li et al. and Huang et al. compared different anti-HER3 agents in lung, head and neck and breast carcinomas combined with either cetuximab (inhibiting-EGFR antibody) and/or trastuzumab (inhibiting-HER2 antibody) and concluded a more prominent anti-tumour response with the two different anti-HER3 agents and combined therapy compared to either agent alone. There might also be an effect of bypassing drug resistance. This suggests the importance of further investigation of combined antibody therapy for clinical use [Citation27,Citation28]. Multiple-targeted therapeutics [i.e. thyrosine kinase inhibitors (TKI's), antibodies, small molecules] that act on either; HER2, HER3 or on downstream targets are reported effective in blocking oncogenic HER3 signalling [Citation29]. An interesting approach in a clinical trial would be to map expression of different members of the HER family in solid tumours when biological treatments are used [Citation12].
Conclusion
We have monitored HER3 through cancer progression and found that a high proportion of primary CRC, lymph node and liver metastases show membranous HER3 expression. There is a correlation between HER3 expression in primary CRC, in lymph node and in liver metastases. HER3 was not prognostic in this small and selected subset of patients. HER3 might be interesting as a future target in treatment of CRC but further studies are required.
Acknowledgements and fundings
We would like to thank associate professor Bengt Isaksson for contributing with CRC liver metastases and comprehensive data in this field and Yunxia Lu for contributing to the statistical analysis. Thanks also to the colleagues at the Pathological Department at Karolinska University Hospital associate professor Göran Elmberger and MD Anna Kwiecinska. This study was possible thanks to support from The Ihre Foundation of the Swedish Society of Medicine, The Juhlin Foundation, Syskonen Svenssons Foundation and The Cancer Research Foundations of Radiumhemmet, Stockholm, regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- World Cancer Research Fund, International. Available from: http://www.wcrf.org. Cited on 15th, June 2014.
- Sjovall A, Jarv V, Blomqvist L, Singnomklao T, Cedermark B, Glimelius B, et al. The potential for improved outcome in patients with hepatic metastases from colon cancer: A population-based study. Eur J Surg Oncol 2004;30: 834–41.
- Maringe C, Walters S, Rachet B, Butler J, Fields T, Finan P, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: A population-based study of patients diagnosed during 2000–2007. Acta Oncol 2013;52: 919–32.
- Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110–8.
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283–301.
- Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman FT, editor. WHO classification of tumours of the digestive system, 4th ed. Lyon: IARC; 2010. pp. 134–46.
- Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 2007;60:1112–6.
- Berg M, Søreide K. Genetic and epigenetic traits as biomarkers in colorectal cancer. Int J Mol Sci 2011;12:9426–39.
- Lee JC, Wang ST, Chow NH, Yang HB. Investigation of the prognostic value of coexpressed ErbB family members for the survival of colorectal cancer patients after curative surgery. Eur J Cancer 2002;38:1065–71.
- Maurer CA, Friess H, Kretschmann B, Zimmermann A, Stauffer A, Baer HU, et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol 1998;29:771–7.
- Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: A meta-analysis. J Natl Cancer Inst 2013;105:266–73.
- Desai MD, Saroya BS, Lockhart AC. Investigational therapies targeting the ErbB (EGFR, HER2, HER3, HER4) family in GI cancers. Expert Opin Investig Drugs 2013; 22:341–56.
- Hsieh A, Moasser M. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer 2007;97:453–7. Review.
- Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther 2008; 15:413–48.
- Yoshioka T, Nishikawa Y, Ito R, Kawamata M, Doi Y, Yamamoto Y, et al. Significance of integrin alphavbeta5 and erbB3 in enhanced cell migration and liver metastasis of colon carcinomas stimulated by hepatocyte-derived heregulin. Cancer Sci 2010;101:2011–8.
- Beji A, Horst D, Engel J, Kirchner T, Ullrich A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin Cancer Res 2012;18:956–68.
- Amin DN, Campbell MR, Moasser MM. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol 2010;21:944–50.
- Campbell MR, Amin D, Moasser MM. HER3 comes of age: New insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res 2010;16:1373–83.
- Lédel F, Hallström M, Ragnhammar P, Ohrling K, Edler D. HER3 expression in patients with primary colorectal cancer and corresponding lymph node metastases related to clinical outcome. Eur J Cancer 2014;50:656–62.
- Wei Q, Shui Y, Zheng S, Wester K, Nordgren H, Nygren P, et al. EGFR, HER2 and HER3 expression in primary colorectal carcinomas and corresponding metastases: Implications for targeted radionuclide therapy. Oncol Rep 2011;25:3–11
- Ljuslinder I, Malmer B, Isaksson-Mettavainio M, Oberg A, Henriksson R, Stenling R, et al.ErbB 1–4 expression alterations in primary colorectal cancers and their corresponding metastases. Anticancer Res 2009;29:1489–94.
- Peeples C, Shellnut J, Wasvary H, Riggs T, Sacksner J. Predictive factors affecting survival in stage II colorectal cancer: Is lymph node harvesting relevant? Dis Colon Rectum 2010;53:1517–23.
- Slesser AA, Georgiou P, Brown G, Mudan S, Goldin R, Tekkis P. The tumour biology of synchronous and metachronous colorectal liver metastases: A systematic review. Clin Exp Metastasis 2013;30:457–70.
- Tan EK, Ooi LL. Colorectal cancer liver metastases – understanding the differences in the management of synchronous and metachronous disease. Ann Acad Med Singapore 2010; 39:719–15.
- Miglio U, Mezzapelle R, Paganotti A, Allegrini S, Veggiani C, Antona J, et al. Mutation analysis of KRAS in primary colorectal cancer and matched metastases by means of highly sensitivity molecular assay. Pathol Res Pract 2013;209:233–6.
- Scartozzi M, Mandolesi A, Giampieri R, Bittoni A, Pierantoni C, Zaniboni A, et al. The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab. Oncologist 2011;16:53–60.
- Li C, Brand TM, Iida M, Huang S, Armstrong EA, van der Kogel A, et al. Human epidermal growth factor receptor 3 (HER3) blockade with U3-1287/AMG888 enhances the efficacy of radiation therapy in lung and head and neck carcinoma. Discov Med 2013;16:79–92.
- Huang J, Wang S, Lyu H, Cai B, Yang X, Wang J, et al. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer 2013;12:134.
- Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell 2013;23:603–17.