Abstract
Objectives. This cross-sectional study estimates the resource use and costs among prevalent colorectal cancer (CRC) patients in different states of the disease.
Methods. Altogether 508 Finnish CRC patients (aged 26–96; colon cancer 56%; female 47%) answered a questionnaire enquiring about informal care, work capacity, and demographic factors. Furthermore, data on direct medical resource use and productivity costs were obtained from registries. Patients were divided into five mutually exclusive groups based on the disease state and the time from diagnosis: primary treatments (the first six months after the diagnosis), rehabilitation, remission, metastatic disease, and palliative care. The costs were calculated for a six-month period. Multivariate modeling was performed to find the cost drivers.
Results. The costs were highest during the primary treatment state and the advanced disease states. The total costs for the cross-sectional six-month period were €22 200 in the primary treatment state, €2106 in the rehabilitation state, €2812 in the remission state, €20 540 in the metastatic state, and €21 146 in the palliative state. Most of the costs were direct medical costs. The informal care cost was highest per patient in the palliative care state, amounting to 33% of the total costs. The productivity costs varied between disease states, constituting 19–40% of the total costs, and were highest in the primary treatment state.
Conclusions. The first six months after the diagnosis of CRC are resource intensive, but compared with the metastatic disease state, which lasts on average for 2–3 years, the costs are rather modest. Informal care constitutes a remarkable share of the total costs, especially in the palliative state. These results form a basis for the evaluation of the cost effectiveness of new treatments when allocating resources in CRC treatment.
The incidence of colorectal cancer (CRC) has been increasing in the Western world. It has been estimated that in Europe around 470 000 new cases were diagnosed in 2012 and CRC was the third most common cancer type after breast and prostate cancer [Citation1]. The number of CRC-related deaths has increased more slowly and was estimated to be around 210 000 per year in 2008. In Finland, the incidence of CRC was 2904 and the prevalence 21 836 in 2012 [Citation2]. At the time of diagnosis, 48% of the patients have local disease and 23% metastatic disease [Citation3]. Currently, 62% of patients survive for at least five years after diagnosis in Finland [Citation2]. The improved survival has increased the number of patients living with a history of CRC.
It has been estimated that in the Nordic countries CRC is the second most expensive cancer per citizen, after breast cancer [Citation4]. It is expected that the CRC costs will increase the most among all cancers. As most cases are diagnosed at the age of 60–80 years, the costs consist mainly of direct healthcare costs.
The increasing costs are driven mainly by the changes in incidence but also by the more advanced and more expensive treatment options used, which lead to prolonged survival and, consequently, to increased prevalence. In order to evaluate whether novel interventions should be implemented, a proper understanding of the costs of treatments as well as their effectiveness in real life is needed.
The aim of this study is to provide essential results for health economic analyses of new interventions and to increase the understanding of the CRC-related costs per patient in different states of the disease. All the different cost elements are considered: direct medical costs, productivity costs, and informal care costs. Furthermore, this study explores the factors associated with higher costs in different states of the disease.
Patients and methods
The study is a cross-sectional survey of CRC patients’ prevalence-based resource use in real life. It was carried out between September 2009 and April 2011 in the Helsinki and Uusimaa Hospital District, which is responsible for secondary care treatment of nearly 1.5 million inhabitants of Southern Finland, representing approximately 30% of the whole Finnish population. The study was approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District and written informed consent was obtained from patients before inclusion. The treatment of patients followed the normal routines and was not affected by the study in any way. This study is part of a research project in which health-related quality of life (HRQoL) and cost data have been collected from 2000 patients suffering from breast, prostate, or CRC [Citation5–7]. The study has been registered in the Helsinki and Uusimaa Hospital District Register (www.hus.fi) with the unique trial number 233895.
Patients
A total of 508 patients were included in the study. Patients were identified from hospital records by the date of diagnosis, after which questionnaires and consent letters were mailed to them. All patients aged 18 years or older and diagnosed with CRC were eligible for the study. Patients were divided into five mutually exclusive groups based on the time from diagnosis and their metastatic status. The costs were calculated for a six-month period and do not represent the whole cost of CRC over time. The states and the time frame were defined according to clinical relevance and to be applicable in health economic modeling. The time frame for the data query was defined by the disease stage and the response date. For patients with local disease, the states were: primary treatments (0–6 months from diagnosis), rehabilitation (6–18 months from diagnosis), and remission (more than 18 months from diagnosis). Patients with metastatic disease were divided into two groups: metastatic disease, in which patients received oncological treatments, and palliative care, in which patients received only palliative treatment. For the palliative care group, the costs were calculated for the whole period after the palliative care decision. The patient selection has been described in more detail elsewhere [Citation5].
To be able to assess the incremental resource use due to CRC data on outpatient medication, the use of private health care services, and days absent from work, in addition to the patients, two population controls were also obtained, matched for age, place of residence, and sex, excluding individuals with a cancer diagnosis. The sample was extracted from the register of the National Social Insurance Institution of Finland (SII) covering outpatient medication, sickness allowances, and private healthcare utilization.
Resource use and cost components
The resource and cost data encompass all the relevant components. The prevalent costs occurring within the defined six-month period were calculated. The data and their sources are described in more detail below. All the costs in Euros were calculated according to the 2010 price level. All other currencies were converted to Euros based on exchange rate of 31 December 2010.
Specialist care
Secondary health care in Finland is organized by municipalities through hospital districts. Data on resource use and costs were extracted retrospectively from hospital records. The data contained information about the exact day on which the hospital visit started and ended, diagnosis and procedure code, and patient level costs, including overheads, equipment, hospitalization, and drugs for inpatient use. Only CRC-related resource use was included and the decision on inclusion was made by a clinical specialist. The costs of travel to the place of treatment were also included if they exceeded the patient maximum co-pay, €14 per visit. The number of journeys and costs were available from the registers of the SII.
Primary health care
In Finland, primary health care is organized at the municipal level. The use of public primary health care services was available from patients’ home municipalities and accessible for 434 patients from the three largest cities in the area, Helsinki, Espoo, and Vantaa, the populations of which make up more than 80% of the total population of the hospital district. The data consist of information concerning GP and nurse visits, home hospice care, and primary care hospitalization. For the 74 patients for whom primary health care data were missing, we used propensity score matching to find a patient whose value we used to replace the missing value, allowing us to calculate the total costs. As the cause of the primary health care visit was not available from the registries, patients were asked to estimate the number of visits due to their CRC within the previous three months. Based on this, we estimated the resource use over the six-month period. Average unit costs for visits and hospital days from Helsinki, Espoo, and Vantaa were used to calculate the costs. Resource use and unit cost data were also available from the local hospice care unit.
Due to the fairly comprehensive provision of public health care in Finland, private health care is usually only complementary. Data on private doctor visits and costs were available from the SII registries. As the diagnosis code for visits was not available, CRC-related visits were calculated by subtracting the mean number of control population private health care visits and health care costs from the number of visits of CRC patients.
Outpatient medication
Outpatient medication in Finland is reimbursed by the SII. Consequently, reliable data on outpatient medication usage and costs were available from the SII registers. The costs of CRC-related medication were established by subtracting the average medication costs of two controls from the costs of the patients.
Productivity costs
Productivity loss occurs when a patient is unable to work normally due to his or her cancer. In the questionnaire, patients were asked whether they had retired from working life due to CRC. The number of working days absent due to CRC was available from the SII registries.
To value the productivity, we used the most commonly employed human capital approach whereby the margin of production of the individual is valued by his or her pre-tax salary [Citation8]. The productivity loss was then calculated by multiplying the number of days absent from work by the average daily labor cost, including the employer's social security payments of on average 38.6% in addition to the pre-tax salary. The daily salary for the patients was calculated from salary-based sickness benefits, available from the SII.
Informal care
Informal care was included based on patients’ own estimates derived from the questionnaires. Patients were asked to recall the number of hours per week for which they had received informal care and support within the previous three months. The estimation was then extrapolated for the whole six-month period. The maximum amount of daily support and care was limited to 16 hours. To be able to calculate the total costs, we used propensity score matching to impute missing values.
The valuation was performed based on the proxy good method [Citation9], which values the time spent on supporting a patient based on the shadow price of a market substitute. We used the mean hourly pre-tax salary of €13.63 for a practical nurse in 2010 [Citation10]. The hourly labor cost was then calculated by adding 38.6% social security payments for employers to the pre-tax salary, which resulted in the final cost of €18.89 per hour, which was then used in the analysis.
Data analysis
The main objective was to estimate the mean cost caused by CRC in five different states of the disease for a six-month period. The differences in the costs and resource use between the states are reported based on confidence intervals.
The log linear multivariate analysis was used to explore how background factors are associated with the total costs. Due to the skewed distribution of the total costs, the dependent variable was the ln (natural logarithm) of the total costs and cohabiting, age, gender, education level, tumor site, and HRQoL score measured by the 15D were the independent variables. The 15D is a generic, 15-dimensional HRQoL instrument providing a preference-based, single index value [Citation11]. The analysis was performed using the fixed-model method. Four different models were built: for the primary treatment state, for the remission and rehabilitation states, for the metastatic disease state, and for the palliative care state.
A risk level of 5% was used for type 1 errors in all the analyses. The analyses were performed with the SPSS 21 software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The age of the patients ranged from 26 to 96 years with a mean of 68 years (). Of the patients, 57% had their primary carcinoma in their colon and 43% in their rectum. Female patients accounted for 47% of the sample. The majority of the patients were married/cohabiting and had received higher education defined as having completed at least high school. The working status differed among the groups: 57% of the patients in the primary treatments group were retired, compared with 72–90% in the other groups. The age and sex distribution did not differ significantly between respondents and non-respondents. The response rate was 61% [Citation5]. The mean duration of palliative care was 181 days. The resource use and costs for the palliative care state were recorded for that period.
Table I. Patient characteristics.
Direct health care costs
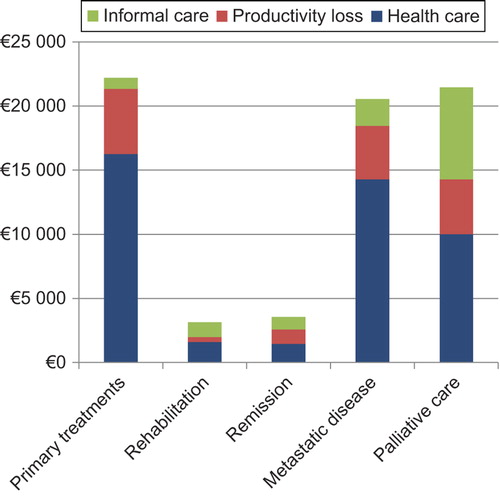
The direct costs per patient over a six-month period varied substantially between disease states. The first six months after diagnosis were the most resource intensive period, followed by the metastatic disease and palliative care states. After the initial six months, the treatment costs were small if the disease did not recur or progress. The direct health care costs, including traveling expenses, over a six-month period were €16 244 in the primary treatment state, €1601 in the rehabilitation state, €1450 in the remission state, €14 277 in the metastatic state, and €10 004 in the palliative state ().
Table II. Direct healthcare costs per patient.
In the primary treatment state, the costs were driven by secondary care inpatient costs (75% of the total costs) as surgery is the standard treatment for these patients. However, due to different techniques and the fact that not all patients are eligible for surgery but some might need a period of intensive care, the cost of hospitalization varied widely between patients, from €0 to 97 000 during the first six months after the diagnosis. The inpatient costs were also high in the metastatic disease group and were mostly driven by the medication administered in hospitals. The total medication cost per patient was €1945 in the primary treatment state, €395 in the rehabilitation state, €272 in the remission state, €8602 in the metastatic disease state, and €246 in the palliative care state.
The costs due to outpatient visits were largest in the metastatic state and in the primary treatment state. In the palliative care state, 10% of the outpatient visits were emergency visits, compared with 4% in the metastatic state, 7% in the rehabilitation state, 4% in the remission state, and 4% in the primary treatment state.
The utilization of primary health care was small and remained stable among CRC patients (€424–640 within the six-month period) in different states of CRC except the palliative care state, in which the cost per patient was €1567. The mean cost of hospice care in the palliative state was €4681 ranging from €0 to €34 626 per patient. Hospice care costs contributed 47% of the total direct costs in that group.
The mean costs of travel to the place of treatment ranged from €37 to €403 between disease states, depending on the number of visits to receive secondary health care.
Productivity costs
The productivity loss caused by CRC was substantial in every state, especially in the primary treatment state and among patients with metastatic disease. Most of the productivity costs were due to sick leave in the early states of the disease and due to early retirement in the metastatic and palliative care states. The cost of early retirement and absence from work was €5098 per patient during the first six months after the diagnosis, €405 in the rehabilitation state, €1130 in the remission state, €4165 in the metastatic state, and €4271 in the palliative care state (). In the primary treatment state, 4.9% of the patients had retired due to CRC, in the rehabilitation state 1.3%, in the remission state 4.6%, in the metastatic state 12.8%, and in the palliative care state 19.4%.
Table III. Productivity loss per patient (six months).
Informal care
The utilization of informal care was most prevalent among patients in the palliative care state (10.2 hours per week) and among those in the metastatic state (4.3 hours), respectively. The estimated cost of informal care per patient during the palliative state was €7184 and for the six-month period in the metastatic state it was €2098. The need for informal care due to cancer was minimal among patients in the rehabilitation and remission states (). Of all the patients, 20% reported that they had received at least some informal care from their family or friends because of CRC. The same proportion was 76% in the palliative care state.
Table IV. Informal care costs per patient (six months).
Total costs
The total costs, including direct health care costs, informal care costs, and productivity losses, were highest in the primary treatment state, totaling €22 200 for the six-month period (). The respective total costs were €2106 in the rehabilitation state, €2812 in the remission state, €20 540 in the metastatic disease state, and €21 460 in the palliative state. The direct health care cost was the largest cost item in all the states, but the costs due to informal care were substantial in the palliative state, constituting 33% of the total costs. Productivity loss caused 23% of the total costs in the primary treatment state, 19% in the rehabilitation state, 40% in the remission state, 20% in the metastatic disease state, and 20% in the palliative state.
Cost drivers
The multivariate regression analysis revealed that background factors can explain only around 9–24% of the variance in the total costs caused by CRC in various disease states (). Within newly diagnosed patients, the costs caused by the treatment of CRC were higher among female patients. This was not the case in the other disease states. A better HRQoL, measured by the 15D, was associated with lower costs in all the states of CRC. This was most evident and statistically significant in the remission and rehabilitation states. The education level and whether the patient was cohabiting were not statistically significantly associated with the total costs. Whether the patient had rectal or colon cancer showed no significant association with the costs. Younger patients’ costs were higher in all the phases of cancer care due to their higher productivity costs.
Table V. Multivariate analysis of cost drivers with the natural logarithm of total costs as the dependent variable.
Discussion
CRC causes considerable costs, but they differ markedly in the various disease states. The total costs as well as the direct costs were highest within the first six months after diagnosis. This was expected as the initial treatment usually involves operative intervention and hospitalization. However, the costs vary greatly from patient to patient as the stage of the disease differs at the time of diagnosis, leading to different therapy regimens and lengths of hospitalization. For the six-month cross-sectional period, the palliative care state was the second most costly phase, mainly due to substantial informal care utilization. In the remission and rehabilitation states, the CRC-related costs were low and mostly associated with productivity loss caused by sick leave and early retirement. The cost of medication was moderate in the other disease states except for the metastatic disease state, in which it contributed more than 60% of all the health care costs.
Productivity loss caused on average 23% of the total CRC-related costs, even though 65% of the patients were above 65 years old. It was driven by sick leave in the early states of the disease, but by early retirement in the later states. As CRC patients are on average relatively old, the total burden constituted by productivity losses is moderate compared with, e.g. breast cancer in which the productivity loss contributed from 61% to 78% of the total costs based on disease state [Citation12]. The costs of informal care have an important impact on the total burden of disease. Informal care constituted on average 11% of the total cost, but as expected it varied greatly between states, being clearly highest in the palliative care state.
Multivariate analysis revealed that a younger age and worse HRQoL are linked with higher CRC- related costs. Among younger patients, the treatments may be more aggressive than in older patients, leading to more intensive health care utilization as well as productivity losses due to sick leave and early retirement. Furthermore, some previous studies have reported that the costs are higher in younger patients [Citation13,Citation14]. HRQoL captures the total effect of treatment-related side effects and functional capacity. It is logical that patients with more symptoms or lower functionality, and consequently lower HRQoL, will need more health care resources. Symptoms could be caused by the disease, but quite often they are treatment related. It is obvious that the disease stage at the time of diagnosis is critical and if the disease relapses or progresses, more costs and resource use occur. If the disease progression could be avoided, it would prevent considerable future costs associated with the metastatic and palliative phases of the disease. Other studies have reported that rectal cancer is more expensive to treat than colon cancer and also that recurrent disease, the need for a stoma, and intensive nursing care are likely to increase the costs [Citation15]. This was not supported by our results.
This is the first study in Nordic countries to evaluate CRC-related resource utilization and costs in different states of the disease in the everyday practice of health care. The study combines various data sources to capture all the cost components. We included inpatient and outpatient resource utilization in both the primary and the specialist care setting and assessed early retirement and sick leave days, as well as informal care given by the patient's family members and friends. The scope was to assess only the costs caused by CRC. To ensure this, we used a matched control population. These kinds of studies are seldom conducted, even though they provide essential information for health economic evaluations of new treatment interventions. The study results could also be used to analyze, e.g. the cost effectiveness of a CRC screening program: screening for CRC has the capacity to both reduce the number of cancer cases, and also the number of patients in metastatic and palliative states of the disease and, therefore, the total costs caused by CRC [Citation16]. However, also opposite results have been reported [Citation17].
There are certain limitations regarding the cross-sectional design of the study. We were not able to calculate the total cost per patient over the whole disease period, nor did the setting allow us to compare the total health care costs for the society between disease states as the number of patients going through treatments for metastatic disease is naturally different from that of patients receiving primary treatments after diagnosis. In this setting, the costs of the intensive primary treatments after diagnosis and palliative care seem to be very high compared with the costs in other states. It should, however, be kept in mind that these phases usually last for only approximately six months, whereas survival with metastatic disease before palliative care usually lasts for 2–3 years. Thus, comparing the six-month costs presented here is not feasible without adjusting the time spent in each state and the probability of disease progression after primary treatments. For example, if we would assume that after six months of primary treatment, and 12 months in the rehabilitation and remission states, a patient's disease would progress so that he would stay 18 months in the metastatic disease state and thereafter receive only palliative care for six months, the total costs of treating the patient's CRC would sum up to €110 198.
Another limitation is that primary care resource utilization data were not available for all the patients and we needed to impute the missing values. This might decrease the reliability of the results. Informal care is difficult to estimate and 20% of the patients had left that question unanswered, so those values were imputed. The recall method used here is not as reliable as a diary-based method, but it is less time consuming. It is also not unambiguous how the valuation of informal care should be carried out [Citation9]. We used the proxy good method, which might underestimate the true value [Citation18]. The travel expenses might also be underestimated as we included only visits for which the cost exceeded the co-pay level of €14.
There are only a limited number of studies available on resource use and costs in CRC. The methods followed to estimate the costs vary and the results are difficult to compare [Citation19]. Most of the studies focused on specific disease states and were based on clinical trials. In the US, several studies have estimated the lifetime health care cost of CRC. The results varied between €21 423 and €75 064 [Citation14,Citation20–22]. The annual health care cost of a prevalent average CRC patient was estimated to range from €4084 to €8692 depending on the register used [Citation23]. The six-month costs in our study were a little higher, ranging from €1450 to €16 244 based on the disease state.
Estimations of costs attributable to the first year after diagnosis vary from €15 117 to €30 784 [Citation13,Citation21,Citation22,Citation24–27]. The health care costs for the first six months and the six-month rehabilitation in our study totaled €17 845, which is in line with the previous results. In a French study, the cost of surveillance was estimated, using a linear mixed model, to be €713 per patient over a three-year period if French guidelines were applied [Citation28]. This is a very low estimate compared with our annual cost of surveillance: rehabilitation plus remission was €3051 per patient but perhaps highlights the difference between real life costs and modeled estimates. In US studies, the cost of 12-month surveillance ranged from €2470 to €3203, which is comparable to our findings [Citation13,Citation21]. A Norwegian study estimated that the lifetime total cost of post-operative surveillance was €20 530 per patient [Citation29]. In the metastatic disease state, cost studies have mainly been performed alongside clinical trials focusing on specific treatment options, making comparison with our results difficult. The mean costs in the palliative care phase have been estimated to be €2109 per month in the USA [Citation30]. The total cost per patient in the last year of life varied from €10 880 to €27 304 [Citation13,Citation14,Citation21,Citation31]. In our study, the direct health care costs in the palliative state were €10 004, which is very close to the estimates in previous studies.
The estimates concerning informal care in CRC are variable. In a US study, the average amount of informal care given to cancer patients within the first two years after diagnosis was 28.1 hours per week [Citation32]. In another US study, the average weekly amount of informal care received was 10.0 hours among over 70-year-old cancer patients receiving treatment at the time for their cancer [Citation33]. Compared with those findings, the amount of informal care that Finnish CRC patients received is rather modest as the average weekly number of caregiving hours was only above 10 hours in the palliative state. The size of the informal care component in cancer-related costs might differ greatly based on the health care system. In a Swedish study exploring costs in several states of breast cancer, the informal care hours varied from 0.8 to 3.8 per week (excluding the palliative state), which were very close to our results [Citation12]. Studies estimating cancer-related productivity costs at the patient level are scarce. The biggest productivity loss caused by CRC is premature mortality, which this study design does not capture.
Conclusion
This study reports the costs that CRC causes in real life in different states of the disease. The costs are substantial, mainly driven by expensive inpatient treatment periods. The study reveals the importance of the informal care received by the cancer patients. Even though the direct health care costs are substantial, the costs of informal care are even higher among patients in the palliative state.
It is important to consider the direct health care cost when evaluating treatment options. However, from the societal perspective, productivity losses and informal care costs should also be taken into account. This study provides cost data from various states of CRC, enabling, e.g. cost effectiveness assessment of a screening program. The development of new, targeted therapies will probably intensify the management of CRC, which will most likely increase the costs, especially in the metastatic disease state. Furthermore, the increasing incidence of CRC will increase the costs caused by the disease. Earlier detection and improved treatments will hopefully lead to improved survival, canceling out the additional costs.
Acknowledgments
We would like to thank Virpi Pelkonen, for her help and support in gathering the clinical data from patient records.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the Cancer Society of Finland and GlaxoSmithKline Oy, Finland. Sponsors were not involved in the study design or in the collection, analysis, or interpretation of data, nor were they involved in the writing of the manuscript or in the decision to submit the manuscript for publication.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. [cited 2014 Jun 27]. Available from: globocan.iarc.fr
- Finnish Cancer Registry, Cancer Statistics, updated on 24/4/2014 [Internet]. [cited 2014 Jun 27]. Available from: www.cancerregistry.fi
- Järvinen H, Kasvaimet LA. In: Färkkilä M, Isoniemi H, Kaukinen K, Puolakkainen P, editors. Gastroenterologia ja hepatologia (in Finnish), 2nd ed. Helsinki: Duodecim; 2013.
- Kalseth J, Halsteinli V, Halvorsen T, Kalseth B, Anthun K, Peltola M. Costs of cancer in the Nordic countries. A comparative study of health care costs and public income loss compensation payments related to cancer in the Nordic countries in 2007. Trondheim: SINTEF Technology and Society; 2011.
- Färkkilä N, Sintonen H, Saarto T, Järvinen H, Hänninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Colorectal Dis 2013;15:e215–22.
- Torvinen S, Färkkilä N, Sintonen H, Saarto T, Roine RP, Taari K. Health-related quality of life in prostate cancer. Acta Oncol 2013;52:1094–101.
- Färkkilä N, Torvinen S, Roine RP, Sintonen H, Hänninen J, Taari K, et al. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual Life Res 2014;23:1387–94.
- Liljas B. How to calculate indirect costs in economic evaluations. Pharmacoeconomics 1998;13:1–7.
- Hoefman RJ, van Exel J, Brouwer W. How to include informal care in economic evaluations. Pharmacoeconomics 2013;31:1105–19.
- Official statistics of Finland. Structure of earnings 2010. Helsinki: Statistics of Finland; 2011.
- Sintonen H. The 15D instrument of health-related quality of life: Properties and applications. Ann Med 2001;33:328–36.
- Lidgren M, Wilking N, Jonsson B, Rehnberg C. Resource use and costs associated with different states of breast cancer. Int J Technol Assess Health Care 2007;23:223–31.
- Lang K, Lines LM, Lee DW, Korn JR, Earle CC, Menzin J. Lifetime and treatment-phase costs associated with colorectal cancer: Evidence from SEER-Medicare data. Clin Gastroenterol Hepatol 2009;7:198–204.
- Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures at the end of life for colorectal cancer decedents. J Womens Health (Larchmt) 2007;16:214–27.
- Macafee DA, West J, Scholefield JH, Whynes DK. Hospital costs of colorectal cancer care. Clin Med Oncol 2009;3:27–37.
- Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603–7.
- Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: Results after nine screening rounds. Scand J Gastroenterol 2004;39:846–51.
- Van den Berg B, Brouwer W, van Exel J, Koopmanschap M, van den Bos GA, Rutten F. Economic valuation of informal care: Lessons from the application of the opportunity costs and proxy good methods. Soc Sci Med 2006;62:835–45.
- Yabroff KR, Borowski L, Lipscomb J. Economic studies in colorectal cancer: Challenges in measuring and comparing costs. J Natl Cancer Inst Monogr 2013;46:62–78.
- Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med 2010;170:537–42.
- Howard DH, Tangka FK, Seeff LC, Richardson LC, Ekwueme DU. The impact of detection and treatment on lifetime medical costs for patients with precancerous polyps and colorectal cancer. Health Econ 2009;18:1381–93.
- Maroun J, Ng E, Berthelot JM, Le PC, Dahrouge S, Flanagan WM, et al. Lifetime costs of colon and rectal cancer management in Canada. Chronic Dis Can 2003;24: 91–101.
- Yabroff KR, Warren JL, Banthin J, Schrag D, Mariotto A, Lawrence W, et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: What is the impact of data source? Med Care 2009;47:S64–9.
- Luo Z, Bradley CJ, Dahman BA, Gardiner JC. Colon cancer treatment costs for Medicare and dually eligible beneficiaries. Health Care Financ Rev 2010;31:35–50.
- Clerc L, Jooste V, Lejeune C, Schmitt B, Arveux P, Quantin C, et al. Cost of care of colorectal cancers according to health care patterns and stage at diagnosis in France. Eur J Health Econ 2008;9:361–7.
- Ramsey SD, Mandelson MT, Berry K, Etzioni R, Harrison R. Cancer-attributable costs of diagnosis and care for persons with screen-detected versus symptom-detected colorectal cancer. Gastroenterology 2003;125:1645–50.
- Bouvier V, Reaud JM, Gignoux M, Launoy G. Cost of diagnostic and therapeutic management of colorectal cancer according to stage at diagnosis in the Calvados Department, France. Eur J Health Econ 2003;4:102–6.
- Lejeune C, Binquet C, Bonnetain F, Mahboubi A, Abrahamowicz M, Moreau T, et al. Estimating the cost related to surveillance of colorectal cancer in a French population. Eur J Health Econ 2009;10:409–19.
- Korner H, Soreide K, Stokkeland PJ, Soreide JA. Systematic follow-up after curative surgery for colorectal cancer in Norway: A population-based audit of effectiveness, costs, and compliance. J Gastrointest Surg 2005;9:320–8.
- Koroukian SM, Beaird H, Madigan E, Diaz M. End-of-life expenditures by Ohio Medicaid beneficiaries dying of cancer. Health Care Financ Rev 2006;28:65–80.
- Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 2008;100: 630–41.
- Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer 2009;115:4362–73.
- Hayman JA, Langa KM, Kabeto MU, Katz SJ, DeMonner SM, Chernew ME, et al. Estimating the cost of informal caregiving for elderly patients with cancer. J Clin Oncol 2001;19:3219–25.