Abstract
Purpose. To report results from the five-year follow-up on a previously reported study using image-guided radiotherapy (IGRT) of localized or locally advanced prostate cancer (PC) and a removable prostate stent as fiducial.
Material and methods. Patients with local or locally advanced PC were treated using five-field 3D conformal radiotherapy (3DRT). The clinical target volumes (CTV) were treated to 78 Gy in 39 fractions using daily on-line image guidance (IG). Late genito-urinary (GU) and gastro-intestinal (GI) toxicities were scored using the radiotherapy oncology group (RTOG) score and the common toxicity score of adverse events (CTC) score. Urinary symptoms were also scored using the international prostate symptom score (IPSS).
Results. Median observation time was 5.4 year. Sixty-two of the 90 patients from the original study cohort were eligible for toxicity assessment. Overall survival, cancer-specific survival and biochemical freedom from failure were 85%, 96% and 80%, respectively at five years after radiotherapy. Late toxicity GU and GI RTOG scores ≥ 2 were 5% and 0%. Comparing pre- and post-radiotherapy IPSS scores indicate that development in urinary symptoms after radiotherapy may be complex.
Conclusions. Prostate image-guided radiotherapy using a prostate stent demonstrated survival data comparable with recently published data. GU and GI toxicities at five-year follow-up were low and comparable to the lowest toxicity rates reported. These findings support that the precision of the prostate stent technique is at least as good as other techniques. IPSS revealed a complex development in urinary symptoms after radiotherapy.
The use of fiducials in the prostate combined with image-guided radiotherapy (IGRT) has demonstrated a significant reduction in positioning uncertainties during prostate radiotherapy, and consequently the potential to reduce setup margins and irradiated volume of normal tissue [Citation1]. Reduced setup margins may be the main explanation for the reduced radiation-induced genito- urinary (GU) and gastro-intestinal (GI) late toxicity observed in several recent studies [Citation2–4]. The reduction in setup margins may compromise treatment efficiency as suggested in some studies [Citation5,Citation6]. Several other studies, however, have reported unchanged or improved biochemical freedom from failure even with reduced setup margins [Citation2–4]. Implanted gold markers (GM) has been the standard choice as fiducial in IGRT of prostate cancer [Citation7]. The use of a nickle titanium removable prostate stent as fiducial for co-registration of planning computer tomography (CT) and magnetic resonance imaging (MRI) and subsequent IGRT treatment has been described in detail earlier [Citation8–12]. Advantages of the prostate stent compared to gold markers were: 1) non-invasive; 2) removable; 3) large 3D object; and 4) good signal i both CT and MR and thus well suited for MR CT co-registration of the prostate. This five-year follow-up of the clinical outcome following image-guided radiotherapy (IGRT) of localized or locally advanced prostate cancer (PC) represents a continuum of an ongoing process evaluating the removable prostate stent as fiducial.
Material and methods
Patients
The original cohort consisted of 90 patients with local and locally advanced prostate cancer. They were treated with radical radiotherapy at the University Hospital of Aalborg Denmark from March 2007 until May 2009. The patient cohort is previously described in detail [Citation11,Citation12]. Exclusion from toxicity assessment was: biochemical failure, inability to read and understand the questionnaires in Danish and death. Biochemical failure was defined as an elevation of prostate-specific antigen (PSA) above nadir plus 2 ng/ml after radiotherapy. The study was in accordance with the standards of the Helsinki Declaration of 1975, as revised in 2000.
Radiotherapy
The clinical target volume (CTV) was defined as the prostate gland, or the prostate gland plus the proximal third part of the seminal vesicles in case of seminal vesicle invasion. The CTV was outlined on CT using the co-registered MRI images. An isotropic CTV to PTV margin of 5 mm was used. External beam radiotherapy (EBRT) was given using a five-field conformal plan (gantry angles at 9°, 90°, 140°, 220° and 270°). A CTV mean dose of 78 Gy in 39 daily sessions was prescribed. Constraints to organs at risk (OAR) and further treatment details are given in [Citation11]. The Brainlab ExacTracTM system was used for daily on-line IG positioning matching the stent with a digital reconstructed radiogram (DRR) from the planning CT. OAR dose volume histograms were exported out of the treatment planning system (TPS) and dosimetry parameters calculated in MatlabTM. Patients with intermediate- and high risk according to the D’Amico classification received neo-adjuvant hormone therapy (AHT) with either LHRH analogues or non-steroidal anti-androgens for three months before irradiation, during irradiation and for a limited period thereafter, usually for one year according to the recommendations in Denmark at time of treatment.
Toxicity assessment
All eligible men were invited for a yearly clinical visit at the department of urology. The visit included a blood sample for PSA measurement and toxicity assessment using the radiation therapy oncology late radiation morbidity scoring schema (RTOG) of GU and GI toxicity. At the five-year follow-up visit this was supplemented with a telephone interview using the national cancer institute common terminology criteria for adverse events (CTC-AE) version 4.0 analogously to our previously reported three-year follow-up [Citation12]. Urinary symptoms included frequency, urgency, incontinence, dysuria, urinary retention and hematuria. Rectal symptoms included diarrhea, fecal incontinence, proctitis, rectal pain and rectal bleeding.
Furthermore urinary toxicity was evaluated using the validated international prostate symptom score (IPSS) patient questionnaire. Patients filled in the questionnaire before prostate stent insertion, two weeks before start of the radiotherapy (baseline) and five years after end of radiotherapy.
Statistics
Survival data and biochemical freedom from failure data was calculated using the Kaplan-Meier method including all 90 patients from the original cohort. With regard to the cancer-specific survival only patients that died from prostate cancer are counted. All other patients are censored at the date of their last visit or the date of eventual non-prostate cancer-related death. Analogously, patients without biochemical failure were censored at the date of last visit or the date of death without evidence of biochemical relapse. Five year RTOG and CTC scores were tested for correlation with Gleason score, T-stage, co-morbidity, seminal vesicles irradiation, smoking, use of statins and polypharmacy using contingency tables and χ2 statistics. Five-year dichotomized RTOG and CTC scores (Grade = 0 and Grade > 0) were tested for dependency of continuous parameters, such as dosimetry parameters, age and prostate volume, using logistic regression. The dependency of RTOG and CTC toxicity scores on baseline IPSS was tested using ANOVA and t-test. Linear regression was used to test dependency of change from baseline at five year versus baseline IPSS.
Results
The median observation time was 5.4 years. Patient characteristics are shown in . Of the original cohort of 90 patients, 10 patients died without biochemical failure. Three patients died of prostate cancer, and a total of 15 patients had biochemical failure. Overall survival (OS), cancer-specific survival (CSS) and biochemical freedom from failure (BFFF) with confidence interval limits are shown in . The variation found concerning the high dose parameters (Dmax and V72Gy) are small (see Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.987355). This is probably due to the standardized prescribed dose and the applied OAR dose constraints in the dose planning. Patient compliance with regard to toxicity assessment was 92% (57/62) for the RTOG score, 96% (60/62) for the CTC score and 92% (57/62) for the IPSS. A graphical representation showing peak toxicity scores are seen in . The figure also includes our previously reported three-year toxicity CTC scores in 73 patients for completeness and comparison of the RTOG scores at three and five years. No grade 3 or higher late toxicity was registered for either RTOG or CTC at any time. RTOG toxicity grades = 2 were 5% and 0% for GU and GI, respectively. The CTC scores in demonstrate that the major adverse toxicities at both three and five years were urinary frequency and urgency with regard to GU toxicity (grade 2) in 13–15% of the patients and rectal bleeding with regard to GI toxicity (only grade 1) in 18% of the patients.
Figure 1. The upper two panels show plot of the frequencies of RTOG scored toxicities in elegible pa-tients at each year in the five year follow-up period. The genito-urinary score frequencies to the left and the gastro-intestinal score frequencies to the right. The lower two panels analogously show the corresponding CTC scores at year three and five in the folllow-up period. RTOG and CTC scores are color coded grade = 0: blue grade = 1: brown grade = 2: green.
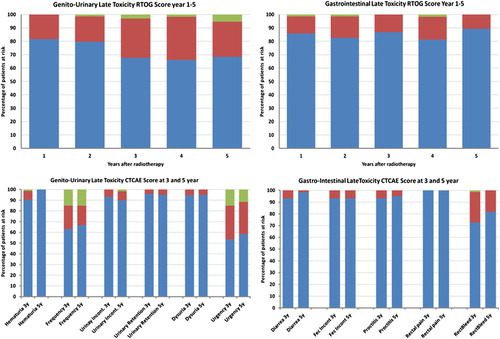
Table I. Patient characteristics of the 60 included patients in the five year toxicity scoring.
Table II. Five-year survival data for the original stent cohort of 90 patients.
Significantly higher RTOG GU toxicity at five years was found related to use of statins (p = 0.005). Similar dependencies were observed for the CTC score at five years regarding urinary frequency (p = 0.02) but not for urinary urgency. The CTC scores for both urinary frequency and urgency significantly correlated to polypharmacy (p = 0.02 and p = 0.03, respectively). No significant dependencies on OAR dosimetry parameters were found for either GU or GI toxicity scores, and no correlation of OAR dosimetry parameters with statin use or polypharmacy was found.
IPSS data including both pretreatment and five-year follow-up results were available for 57 patients. A significant correlation between baseline IPSS and the RTOG GU score and CTC urinary frequency and urgency scores was found as shown in . also shows an overall statistically significant improvement in urinary symptoms at five-year follow-up compared to pretreatment based on the IPSS.
Table III. Baseline International prostate symptom score (IPSS) correlation with 5 year toxicity scores.
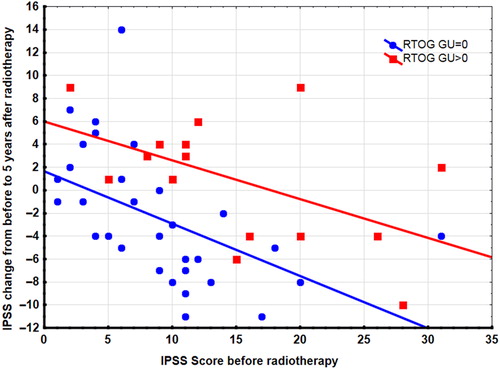
shows a plot of the change in IPSS at five-year follow-up from the baseline IPSS versus the baseline score. Linear regression showed a statistically significant lower IPSS change with increasing baseline IPSS after radiotherapy. demonstrates that the change in IPSS versus baseline IPSS was significantly lower for patients with no toxicity symptoms (RTOG score = 0) compared to patients with toxicity symptoms (RTOG score > 0), but the decrease in change was similar for the two groups.
Figure 2. Changes in IPSS at five year after radiotherapy from pretreatment IPSS plotted versus pre-treatment IPSS. Linear regression lines and data points are shown for two groups: one group with RTOG toxicity score = 0 (Blue), another group with RTOG toxicity score > 0 (Red).
No significant correlations were found concerning RTOG GI toxicity or CTC score for rectal bleeding.
Discussion
This paper reports the five-year follow-up results from the first clinical study using a prostate stent as fiducial during IGRT for prostate cancer. The survival data (OS: 85%, CSS 96% and BFFF 80%) are comparable with the data reported by the RTOG 9406 study (78 Gy and a 3D conformal technique) [Citation13]. They found OS at 85%, CSS at 99% and BFFF at 80%. However, the RTOG 9406 study had a lower fraction of high risk patients compared to our study (31% vs. 71.7% in our study cohort). Comparing survival data normally requires that the risk group frequencies are similar.
Data on BFFF for high risk patients have been comprehensively reviewed using highly standardized inclusion criteria's in a recent study [Citation14]. This review reported five-year BFFF in the range of 30–72% for high risk patients. Compared to this review our results are in the upper end of the reported range. Data from another Danish radiotherapy center has recently been reported [Citation4]. This retrospective study compared 3D conformal with a 10 mm setup margin with IMRT combined with IGRT and a 5 mm setup margin for high risk patients. Only the three-year BFFF is reported; 86% for the 3D conformal and 90.3% for the IMRT treatment. This corresponds well to the previously reported three-year BFFF of 89% for the cohort in this study [Citation12]. Finally, a Canadian study also using a radiation dose of 79.8 Gy and IG 3D conformal treatment with a margin of 10 mm demonstrated five-year BFFF of 77.9% for high risk patients comparable to the 77% found in this study [Citation15]. To summarize the BFFF data using the prostate stent as fiducial are comparable with other studies using similar dose and IGRT techniques. Consequently, it may be concluded that the use of a prostate stent yields at least equal precision as gold markers.
The five-year RTOG GU and GI late toxicity grade ≥ 2 in this study were, respectively, at 5% and 0%. These frequencies are in the low end compared to the findings published in a recent review, where scores grade ≥ 2 were ranging from 5.7% to 41% and 4–33% for GU and GI toxicity, respectively [Citation16]. The best studies applying intensity modulated radiotherapy (IMRT) in this review had a five-year mean frequency of RTOG score grade ≥ 2 of 15.5% (range 7–28.3%) and 10% (range 4–21%), respectively, for GU and GI toxicity. Data from the recently published Danish study has demonstrated a significant improvement in late toxicity at two-year observation time using IG-IMRT with a 5 mm setup margin as compared to 3D conformal radiotherapy using a 10 mm setup margin [Citation4]. They reported RTOG grade ≥ 2 toxicity frequencies, respectively, for GU and GI for IG-IMRT of 29.7% and 5.8%, as compared to analogous frequencies of 41.8% and 57.3% for 3D conformal technique [Citation4]. Consequently, the five-year RTOG grade ≥ 2 scores in the present study, applying a 3D conformal technique with 5 mm setup margin, were at least comparable to the toxicities for the IMRT technique reported from this other Danish center. The RTOG grade ≥ 2 toxicities in the present study are equivalent with the five-year RTOG grade ≥ 2 of 10% and 1.6% for GU and GI, respectively, reported in another recent study using IG-IMRT techniques [Citation2]. Even though toxicity frequencies seem to lower using IG-IMRT techniques and small setup margins, these results are still controversial. Other studies have shown no effect of reducing the setup margin from 10 to 5 mm and reported similar and excellent five-year RTOG grade ≥ 2 toxicities of 6.6–7% and 1.2–2.6% for GU and GI, respectively [Citation17]. The exclusion of patients with BF from the toxicity analysis was to avoid interference in toxicity measures from either disease recurrence or salvage therapy.
To summarize the present study has demonstrated very low GU and GI toxicity frequencies, but a caveat in the present study was a relatively small number of patients and the competing risks of death or BF, which may have led to underestimating five-year toxicity frequencies.
The RTOG and CTC scores in this study gave consistent results with regard to hematuria and rectal bleeding. Only grade = 1 toxicity was observed. An improvement in both hematuria and rectal bleeding from three to five years was observed as have been observed by others [Citation18,Citation19]. The complete clearance of hematuria from three to five years, however, seems unrealistic and is most probably an example of the competing risk of death. In general the CTC scores for urinary frequency and urgency were not consistent with the RTOG GU score at neither three nor five years. This inconsistency was probably due to increased frequency and urgency symptoms requiring α-blocker medications, which scores as grade 2 in CTC only.
Unexpectedly, the use of statin significantly correlated with more adverse urinary toxicities estimated on both RTOG GU scores and CTC scores at five years. The observation of increased urinary toxicity seems, most likely, to be a confounder effect, as the use of statin also correlated significantly to pre-treatment urinary toxicity estimated on IPSS and poly-pharmacy. Unfortunately, no pre-treatment scores existed for either RTOG or CTC in the present study. However, a pre-treatment (baseline) IPSS value has been recorded for most patients in the original cohort [Citation11]. The five-year CTC and RTOG toxicity scores of urinary frequency and urge were significantly related to the baseline IPSS, again indicating that pre-treatment morbidity could have influenced the scoring at five-year follow-up. Actually, this study demonstrated that a significant overall improvement in IPSS urinary symptoms occurred at the five-year follow-up as compared to the pre-treatment IPSS level. Such an effect has been described by others [Citation20]. Furthermore, change in IPSS at five years compared to pre-treatment levels demonstrated improvement in urinary symptoms with increasing pre-treatment IPSS as demonstrated by the regression line in . This improvement was counteracted by manifest radiotherapy toxicity as may be seen by the upward shift of the regression line for patients with a RTOG toxicity score. Consequently, the urinary symptoms observed in this study indicate that development in urinary symptoms after radiotherapy may be more complex as counter acting effects after radiotherapy may occur over time. Such effect may complicate things when urinary late toxicity from different studies are compared. It may also explain why correlation of urinary toxicity to radiation dose may often be elusive. We recommend that future monitoring of urinary toxicity should include pre-treatment and follow-up IPSS in patients treated for localized prostate cancer with modern radiotherapy.
Conclusion
A new technique using a prostate stent as fiducial demonstrated survival data comparable with other published data. Genito-urinary and gastro-intestinal toxicities at five-year follow-up were low and comparable to lowest reported toxicities in the literature. These findings support that the precision of the prostate stent technique is at least as good as other techniques. IPSS revealed a complex development in urinary symptoms after radiotherapy.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.987355.
ionc_a_987355_sm2680.pdf
Download PDF (46 KB)Acknowledgments
The study received foundation from “The IGRT project fund” at Aalborg University Hospital and from CIRRO, the Lundbeck Foundation Centre for Interventional Research in Radiation Oncology. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Zhang M, Moiseenko V, Liu M, Craig T. Internal fiducial markers can assist dose escalation in treatment of prostate cancer: Result of organ motion simulations. Phys Med Biol 2006;51:269–85.
- Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;84:125–9.
- Kok D, Gill S, Bressel M, Byrne K, Kron T, Fox C, et al. Late toxicity and biochemical control in 554 prostate cancer patients treated with and without dose escalated image guided radiotherapy. Radiother Oncol 2013;107:140–6.
- Sveistrup J, af Rosenschold PM, Deasy JO, Oh JH, Pommer T, Petersen PM, et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol 2014;9:44.
- Engels B, Soete G, Verellen D, Storme G. Conformal arc radiotherapy for prostate cancer: Increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys 2009;74:388–91.
- Heemsbergen WD, Al-Mamgani A, Witte MG, van Herk M, Lebesque JV. Radiotherapy with rectangular fields is associated with fewer clinical failures than conformal fields in the high-risk prostate cancer subgroup: Results from a randomized trial. Radiother Oncol 2013;107:134–9.
- Moman MR, van der Heide UA, Kotte AN, van Moorselaar RJ, Bol GH, Franken SP, et al. Long-term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother Oncol 2010;96:38–42.
- Carl J, Nielsen J, Holmberg M, Hojkjaer LE, Fabrin K, Fisker RV. A new fiducial marker for image-guided radiotherapy of prostate cancer: Clinical experience. Acta Oncol 2008;47:1358–66.
- Carl J, Lund B, Larsen EH, Nielsen J. Feasibility study using a Ni-Ti stent and electronic portal imaging to localize the prostate during radiotherapy 1. Radiother Oncol 2006; 78:199–206.
- Korsager AS, Carl J, Ostergaard LR. MR-CT registration using a Ni-Ti prostate stent in image-guided radiotherapy of prostate cancer. Med Phys 2013;40:061907.
- Carl J, Nielsen J, Holmberg M, Larsen EH, Fabrin K, Fisker RV. Clinical results from first use of prostate stent as fiducial for radiotherapy of prostate cancer. Acta Oncol 2011;50:547–54.
- Sander L, Langkilde NC, Holmberg M, Carl J. MRI target delineation may reduce long-term toxicity after prostate radiotherapy. Acta Oncol 2014;53:809–14.
- Michalski J, Winter K, Roach M, Markoe A, Sandler HM, Ryu J, et al. Clinical outcome of patients treated with 3D conformal radiation therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat Oncol Biol Phys 2012;83:e363–70.
- Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. Br J Urol Int 2012;109(Suppl 1):22–9.
- Martin JM, Bayley A, Bristow R, Chung P, Gospodarowicz M, Menard C, et al. Image guided dose escalated prostate radiotherapy: Still room to improve. Radiat Oncol 2009;4:50.
- Ohri N, Dicker AP, Showalter TN. Late toxicity rates following definitive radiotherapy for prostate cancer. Can J Urol 2012;19:6373–80.
- Crehange G, Mirjolet C, Gauthier M, Martin E, Truc G, Peignaux-Casasnovas K, et al. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiother Oncol 2012;103:244–6.
- Syndikus I, Morgan RC, Sydes MR, Graham JD, Dearnaley DP. Late gastrointestinal toxicity after dose-escalated conformal radiotherapy for early prostate cancer: Results from the UK Medical Research Council RT01 Trial (Isrctn47772397). Int J Radiat Oncol Biol Phys 2010;77:773–83.
- Fonteyne V, Villeirs G, Lumen N, De Meerleer G. Urinary toxicity after high dose intensity modulated radiotherapy as primary therapy for prostate cancer. Radiother Oncol 2009;92:42–7.
- Malik R, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer: Urinary outcomes for men with high International Prostate Symptom Scores (IPSS). Int J Radiat Oncol Biol Phys 2011;80:1080–6.
