Abstract
Objective. To evaluate from a planning point of view the dose distribution of adaptive radiation dose escalation in head and neck squamous cell carcinoma (HNSCC) using 18F-Fluoroazomycin arabinoside (FAZA) positron emission tomography/computed tomography (PET-CT).
Material/methods. Twelve patients with locally advanced HNSCC underwent three FAZA PET-CT before treatment, after 7 fractions and after 17 fractions of a carboplatin-5FU chemo-radiotherapy regimen (70 Gy in 2 Gy per fraction over 7 weeks). The dose constraints were that every hypoxic voxel delineated before and during treatment (newborn hypoxic voxels) should receive a total dose of 86 Gy. A median dose of 2.47 Gy per fraction was prescribed on the hypoxic PTV defined on the pre-treatment FAZA PET-CT; a median dose of 2.57 Gy per fraction was prescribed on the newborn voxels identified on the first per-treatment FAZA PET-CT; a median dose of 2.89 Gy per fraction was prescribed on the newborn voxels identified on the second per-treatment FAZA PET-CT.
Results. Ten of 12 patients had hypoxic volumes. Six of 10 patients completed all the FAZA PET-CT during radiotherapy. For the hypoxic PTVs, the average D50% matched the prescribed dose within 2% and the homogeneity indices reached 0.10 and 0.12 for the nodal PTV 86 Gy and the primary PTV 86 Gy, respectively. Compared to a homogeneous 70 Gy mean dose to the PTVs, the dose escalation up to 86 Gy to the hypoxic volumes did not typically modify the dose metrics on the surrounding normal tissues.
Conclusion. From a planning point of view, FAZA-PET-guided dose adaptive escalation is feasible without substantial dose increase to normal tissues above tolerance limits. Clinical prospective studies, however, need to be performed to validate hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma.
Tumor hypoxia is a common feature in head and neck squamous cell carcinoma (HNSCC), which impact on outcome after radiotherapy treatment [Citation1–3]. Several therapeutic interventions specifically targeted toward hypoxic tumors have been proposed, and among them the use of hypoxic cell sensitizers has been shown to significantly improve radiotherapy outcome [Citation4–8]. Radiation dose escalation is another complementary alternative to increase hypoxic cell kill, which has been already proposed by few authors [Citation9,Citation10]. Although attractive, such approach is potentially limited by an increase risk of late toxicity when higher dose delivery on large volumes increases above the tolerance of surrounding normal tissues. Hence, the concept of tailored dose increment, i.e. the so-called ‘dose painting’, aiming at specifically boosting the radiation dose to those hypoxic voxels individualized by molecular imaging [Citation11–14].
Several methods have been developed to detect tumor hypoxia, and among them the use of positron emission tomography (PET)-labeled nitroimidazole compounds is particularly suited for quantitative and repetitive hypoxia determination in humans [Citation15]. Non-invasive imaging methods also allow a precise determination of the spatial distribution of hypoxia. Among various available PET tracers, 18F-fluoroazomycin arabinoside (18F-FAZA) has several advantages such as a straightforward production with high specific activity, a chemical stability after injection, a specific metabolism in hypoxic cells and a rapid clearance of unbound tracer from non-hypoxic tissues leading to high tumor-to-background ratios compared to other tracers [Citation16,Citation17]. In several studies, pre-treatment signal-to-background or signal-to-muscle ratios between 1.2 and 2 have been reported in series of patients with HNSCC [Citation18–23]. In a small series of 12 patients, our group has observed hypoxia in 10 tumors (80%) with SUVmax ranging from 1.8 to 2.9 in the primary tumors, and hypoxic volume up to 54% of the whole tumor [Citation24]. Interestingly, as also reported by others, the hypoxic fraction varied over the course of a radiotherapy treatment, both from a spatial and a quantitative point of view, thus calling for repetitive assessment and eventually dose adaptation during treatment [Citation19,Citation25–29].
In this framework, the objective of this study was to evaluate the dosimetric feasibility of hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma using the previously reported series of patients [Citation24].
Material and methods
Patients
Patients with stage II, III or IV HNSCC treated with concurrent chemo-radiotherapy were selected for this study. Twelve patients (9 men, 3 women) with a median age of 64 years (age range: 54–71 years) were included between November 2012 and July 2013 (Supplementary Appendix 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.990109). The patient work-up included clinical fiberoptic examination, endoscopy under general anesthesia with biopsy of any suspected mucosal sites, contrast enhanced computed tomography (CT) or MRI (magnetic resonance imaging), esophageal endoscopy, dental examination with appropriate tooth extraction if required and 18F-FDG PET-CT. The study was approved by the Institutional Ethics Review Board, and written informed consent was obtained from all patients. Patients were treated with intensity modulated radiation therapy (IMRT) with a total median dose of 70 Gy delivered in 35 daily fractions of 2 Gy over seven weeks on the PTV associated with the primary tumor and the positive nodes. The PTV associated with the non-invaded neck node levels received a median dose of 50 Gy delivered in 25 fractions of 2 Gy over five weeks. Carboplatin-5FU chemotherapy (carboplatin, 70 mg/m2 per day for 4 days and continuous infusion of 5-fluorouracil 600mg/m2 per day for 4 days) was given concomitantly during Weeks 1, 4 and 7 of radiotherapy [Citation30]. For Patients 1 and 3 the use of cetuximab was preferred owing the poorer general status; a loading dose of 400 mg/m2 of cetuximab injected one week prior to radiotherapy followed by weekly doses of 250 mg/m2 were delivered [Citation31]. All patients received the dose of radiation, chemotherapy or cetuximab per protocol.
18F-FAZA production, and PET-CT and planning CT acquisition
18F-FAZA was manufactured according to EU GMP regulations as previously described [Citation24]. 18F-FAZA PET-CT scans were acquired on a time-of-flight (TOF) Gemini TF PET-CT (Philips Medical Systems, Cleveland, OH, USA). Non-fasting patients received 381 (range 345–386) MBq FAZA and were scanned 120 (range 119–150) minutes post-injection for 10 minutes. PET-CT acquisitions (1 bed position) were performed with the patients immobilized on a flat table-top with the head-neck-shoulder immobilization system used for treatment. The PET data were reconstructed with the 3D line of response-TOF blob-based OSEM algorithm from Philips with three iterations and 33 subsets. The voxel size was 2 mm × 2 mm × 2 mm. Planning CT were acquired on a Toshiba scanner in supine position using a thermoplastic immobilization mask. A 2 mm thin section planning CT scanner with IV contrast medium was acquired for all patients.
FAZA PET-CT scan were performed prior to the start of radiotherapy treatment (FAZA PET-CT H0, typically 2–7 days before the start), 1 hour after the delivery of the seventh fraction (FAZA PET-CT H7) and 1 hour after the delivery of the 17th fraction (FAZA PET-CT H17) during radiotherapy.
FAZA image analysis
In all patients, the gross tumor volume for the primary tumor (GTVCT T) and the involved lymph nodes (GTVCT N) were delineated on the planning CT using the CMS treatment planning software (Elekta Computerized Medical System, version 4.64.00, Stockholm, Sweden). Involved lymph nodes were defined as those with a diameter perpendicular to the longest axis above 1 cm. The FAZA PET-CT images were then registered (deformable registration) with the planning CT using the Mim Maestro registration software (Mim Maestro version 6.1, MIM software Inc, Cleveland, OH, USA). For each patient and for each PET-CT, the standardized uptake values (SUV) of FAZA in the GTVT-CT, in the GTVN-CT and in a background region (i.e. 10 consecutive sections in the contralateral posterior neck muscles around the 3rd and 4th cervical vertebra) were determined with PMOD (PMOD technologies, Adliswil, Switzerland, version 3.4). Hypoxic voxels were defined as voxels contained in the GTVCT with a SUV equal to or above the mean SUV value of the background region plus three standard deviations.
Treatment planning
Standard target volumes were manually contoured. GTVCT T and GTVCT N were defined as macroscopic primary tumor and/or pathological lymph nodes. The clinical target volumes (CTV) were defined as the volume of tissue containing the GTV and subclinical malignant disease extension; they were split into CTVhigh and CTVlow risk [Citation32,Citation33]. Two planning target volumes (PTV) were generated: PTV low risk (PTV56Gy) and PTV high risk (PTV70Gy). There were generated by a 4 mm isotropic expansion around the low-risk and high-risk CTVs. Organs at risk (OAR) included the spinal cord and its planning at risk volume (PRV, i.e. a 4-mm extension of the OAR), brainstem and its PRV (4-mm extension of the OAR), parotids and oral cavity.
For each patient and for each PET-CT acquisition, the so-called hypoxic volumes (GTVPET H) were then delineated for the primary tumor (GTVPET T H) and the node(s) (GTVPET N H) by assembling the voxels contained in the GTVCT with a SUV equal to or above the mean SUV value of the background region plus three standard deviations. This operation was done on the PET-CT scans acquired before (H0) and during radiotherapy (H7 and H17). At the seventh fractions, only hypoxic voxels, which did not exist before the start of treatment called ‘newborn voxels’ were included into the GTVPET H7. A similar analysis was performed on the PET-CT performed after the 17th fraction. A 2.5 mm isotropic expansion of the GTVPET T H and the GTVPET N H resulted in corresponding PTVPET H [Citation34].
For dose planning, the goal was to deliver a fixed dose of 86 Gy to any voxels defined as hypoxic during the course of treatment, i.e. before treatment, after the seventh fraction, and after the 17th fraction. To achieve such goal, the treatment planning was divided into three phases optimized separately: phase 1 (fractions 1–7) used pre-treatment 18F-FAZA PET-CT scans, while phase 2 (fractions 8–17) and phase 3 (fractions 18–35) used per-treatment 18F-FAZA PET-CT scans acquired after the seventh and the 17th fractions, respectively (). Treatment planning was performed on a TomoTherapy TPS (Accuray®) with a GPU architecture. Unless otherwise mentioned, the TPS parameterization of Deveau et al. was followed (slice width of 1 cm, modulation factor of 3.0 and a pitch of 0.43). The dose calculation grid was set in ‘fine’ mode (i.e. around 2 × 2× 2 mm3 resolution) [Citation35].
Figure 1. Design of the adaptive dose escalation procedure. For each planning phase, separate image sets (i.e. PET CT H0, PET CT H7 and PET CT H17) were acquired. Using deformable image co-registration, regions-of-interest were deformed from one PET-CT to the next and manually adjusted when needed. For each phase, a new treatment plan was made only on the newborn voxel of that phase (i.e. at the 7th and 17th fraction). The doses were summed anti-chronologically on the pretreatment PET-CT.
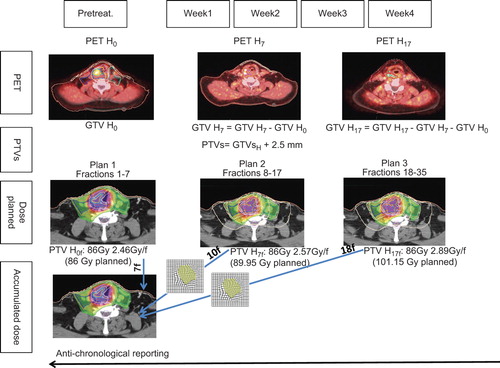
For the phase 1, a median dose of 56 Gy was planned on the PTV56Gy T and the PTV56Gy N, and a median dose of 70 Gy was planned on the PTV70Gy T and the PTV70Gy N using a simultaneous integrated boost technique. A median dose of 86 Gy was planned on the PTV PET T H0 and the PTVPET N H0 using 35 fractions of 2.46 Gy.
For the phase 2, the GTVs, CTVs and OARs delineated on the pretreatment FAZA PET CTH0 were deformed by deformable image co-registration (Maestro registration software version 6.1, MIM software Inc.) onto the per-treatment FAZA PET CTH7, visually inspected, and adjusted when necessary; PTVs and PRVs were then created by expending the CTVs and OARs and they were used for the second planning. For this phase 2, a median dose of 56 Gy on the PTV56Gy T and the PTV56Gy N, and a median dose of 70 Gy on the PTV70Gy T and the PTV70Gy N were planned using a simultaneous integrated boost technique. A median dose of 89.25 Gy was planned on the PTV PET T H7 and PTVPET N H7 with dose per fraction of 2.57 Gy. A median dose of 86 Gy was still planned on PTVPET T H0 and PTVPET N H0 using fractions of 2.46 Gy.
For the phase 3, a similar procedure than for the phase 2 was used, using the per treatment FAZA PET CTH7 which were deformed onto the second per-treatment FAZA PET CTH17,. For this phase 3, we planned a median dose of 56 Gy to the PTV56Gy T and the PTV56Gy N and a median dose of 70 Gy to the PTV70Gy T and PTV70Gy N using a simultaneous integrated boost technique. A median dose of 101.15 Gy was planned on the PTV PET T H17 and on the PTVPET N H17 using dose per fraction of 2.89 Gy. A median dose of 86 Gy was still planned on PTVPET T H0 and PTVPET N H0 using fractions of 2.46 Gy; a median dose of 89.25 Gy was still planned on the PTV PET T H7 and PTVPET N H7 with dose per fraction of 2.57 Gy.
Last, to compare the dose distributions of the escalated dose plans, standard plans with homogeneous adapted 70 Gy on the PTV70Gy T and PTV70Gy N were also created for each patient.
For each phase, the following dose-volume constraints were used in the optimization process: for PTVs, D90% ≥ 99% of the prescribed dose, D95% ≥ 95% of the prescribed dose, D107% < 5% of the prescribed dose; for PRV spinal cord and PRV brain stem, D2% < 30 Gy; for the contralateral parotid, Dmean ≤ 26 Gy; for the ipsilateral parotid, Dmean ≤ 30 Gy. Dose prescription, recording and reporting were performed according to ICRU 83 recommendations [Citation36].
Accumulated dose and reporting
Anti-chronological dose summation was then performed with deformed and adjusted PTVs, OARs and PRVs from the PET CT H17 into the PET CT H0. Eighteen fractions of 2.89 Gy (phase 3, fractions 18–35) and 10 fractions of 2.57 Gy (phase 2, fractions 8–17) were added on the 7 fractions of 2.46 Gy (phase 1, fractions 1–7), such as each hypoxic voxel got a total dose of 86 Gy. Dose metrics used to evaluate and report the plans included D2%, D50%, D95%, D98% and D107% for the PTVs, D2% for the PRV spinal cord and the PRV brainstem, and mean doses to the parotid glands and the oral cavity. In addition, homogeneity indices (HI) were calculated as (D2%–D98%)/D50% as proposed in the ICRU report #83 [Citation36].
Results
On the pre-treatment PET-CT, 10 of 12 patients showed a clear uptake of 18F-FAZA, eight patients in the primary tumor and in the involved node, and two patients in the involved node only (). During treatment, FAZA PET-CT scans were performed in seven patients at fraction #7 and in six patients at fraction #17. Three patients did not get FAZA PET-CT during treatment, i.e. two patients for refusal, and one for technical difficulty due to a precarious respiratory status. When looking at non-hypoxic voxels at pre-treatment examination becoming hypoxic after fraction #7 (so called newborn hypoxic voxels), Seven patients had newborn hypoxic voxel either in the primary tumor or in the involved nodes or both. However, after fraction #17, only three patients still had newborn voxels. When compared with the total hypoxic volume (data not shown), newborn hypoxic voxels represented from 20% to 90% of this volume [Citation24].
Table I. Primary tumor (top) and involved node (bottom) volumes (in ml) assessed by CT and FAZA-PET before and during treatment after fraction #7 and #17. During treatment only the so-called newborn FAZA-PET volumes are indicated.
For each phases of the treatment plans, the dose distribution in the PTVs were in line with the dose prescriptions and respected the ICRU report #83recommendations ( and Supplementary Appendix 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.990109). The average combined dose distributions in the various PTVs are reported in . For the prophylactic nodal irradiation, the average D50% matched the prescribed doses. The HI reached 0.36 and 0.20 for the ipsilateral and contrateral nodal PTVs, respectively, reflecting the slight overlap and/or proximity between the prophylactic PTVs and the high dose PTVs. For the hypoxic PTVs, the average D50% matched the prescribed dose within 2% and the HI reached 0.10 and 0.12 for the nodal PTV 86 Gy and the primary PTV 86 Gy, respectively. For the PTVs 70 Gy (non-hypoxic PTVs), conformity indices reached 0.22, 0.26 and 0.11 for the primary tumor PTVs, the ipsilateral node PTVs, and the contralateral node PTVs, respectively. These figures reflect the slight overlap between the PTVs 70 Gy and the PTVs 86 Gy.
Table II. Added dose distribution in the planning target volumes.
Dose metrics for various organs at risk are reported in for various normal tissues and for each individual patient. Compared to a homogeneous 70 Gy mean dose to the PTVs, the dose escalation to the hypoxic volumes up to 86 Gy did not typically modify the dose metrics. Even in those patients where larger dose differences were observed (e.g. PRV spinal cord and/or PRV brain stem for patients #8, 10 and 12), the dose distribution did not reach the tolerance of these tissues to ionizing radiation.
Table III. Comparison between the dose distribution of a 70 Gy homogeneous plan and a 86 Gy combined plans for various OARs and PRVs for each patient included in the study.
Discussion
Before testing the concept of adaptive radiation dose painting and escalation in a phase I–II trial, several methodological and conceptual issues needed to be matured.
First, in our previous paper, we extensively discussed how hypoxic voxels were defined, and readers are referred to it for further explanations [Citation24]. In short, it may well be that owing our definition of hypoxic voxels, we slightly overestimated the number of hypoxic voxels.
Second, in the present study, it was decided to homogeneously prescribe a dose of 86 Gy to the hypoxic voxels throughout the course of treatment. Why a dose of 86 Gy whereas planning studies with dose up to 105 Gy have been reported in hypoxia dose painting without exceeding the normal tissue tolerance dose [Citation37]? In a phase-I dose searching study aiming at increasing the median radiation dose on FDG-positive voxels in locally advanced HNSCC, the group of DeNeve et al. reported that delivering a median dose of 85.9 Gy was feasible, although 36% of patients (5 patients of 14) developed late mucosal ulceration [Citation38]. It should however be noted that in the latter study, the high-dose volumes (delineated based on FDG-PET) were larger than in our study, and that dose inhomogeneity from 2.5 to 3.5 Gy per fraction were delivered [Citation39]. Why was the dose homogeneously distributed on the hypoxic voxels in our study and not graded based on the intensity of hypoxia? In a rat rhabdomyosarcoma model, a correlation between the pO2 values measured by Electron Paramagnetic Resonance (EPR) and the intensity of FAZA uptake has been established [Citation40]. However, such correlation does not exist in human situation, and it is thus not possible to translate the FAZA-PET signal intensity into an absolute pO2 value. Even if it was possible, when considering the steep radiation dose-response curve as a function of the pO2, it's likely that for voxels with hypoxia a little above the background threshold of FAZA PET intensity, an additional dose of 16 Gy (from 70 to 86 Gy) may likely influence cell kill; on the contrary, for extremely hypoxic voxels, such dose increment will probably have a little effect because of the large oxygen enhancement ratio (OER) at such high level of hypoxia. In other words, radiation dose escalation to counteract cellular hypoxia is likely to be only effective for moderate hypoxia, at least with low-LET radiation. Altogether, these uncertainties have prompted us to test a dose increment aiming at delivering as much dose as feasible to the hypoxic voxels. Other dose painting strategies aiming at redistributing the dose based on the hypoxia distribution and on tumor control probability (TCP) models have been proposed [Citation27,Citation41]. All these strategies need to be validated in clinical studies.
Third, variations in both the intensity and the location of hypoxia within the tumor volume throughout the course of radiotherapy have been reported in the present study and by others [Citation24,Citation28]. This prompted us to perform adaptive planning aiming at delivering a dose of 86 Gy to any voxels that expressed hypoxia in the course of treatment. In this framework, an issue, which is not entirely solved, is how to report the dose that has been delivered. Should the summed dose be reported chronologically on the last planning CT (thus dealing with reporting dose on ‘voxels not existing any longer’), or should the dose be reported anti-chronologically on the first planning CT (thus requiring to ‘re-create disappeared voxels’). For target volume dose reporting, as tumor cells are doomed to progressively disappear during treatment, anti-chronological dose reporting has been favored [Citation42]. For normal tissue dose reporting in adaptive radiotherapy, chronological reporting on the last image that may be the basis for subsequent follow-up images has been proposed [Citation42]. Another approach to sum various plans is to report the dose on an average anatomy model [Citation43]. Whatever the approach, orphans and newborns voxels will be created, thus introducing uncertainties in dose reporting. For example, for patient #12 of the present study, although the planning of each phases of the treatment fulfilled the dose-constraints, in the summed plan, the coverage was no optimal with a worsening of the D98% and D95% of the tumor PTV. This suggests that the validation must be done phase-by-phase, especially for target volumes.
In summary, our study indicated that in HNSCC, from a planning point of view, adaptive dose escalation up to 86 Gy on FAZA PET positive voxels was feasible without substantial dose increase to normal tissues. This concept however needs to be prospectively evaluated in clinical studies both from the efficacy and the toxicity endpoint.
Supplementary material available online
Supplementary Appendix 1–2 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.990109.
ionc_a_990109_sm9053.zip
Download Zip (26.8 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol 2006;24:727–35.
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 2005; 77:18–24.
- Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: Evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 1991;51:3316–22.
- Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: Results of a phase III randomized trial. J Clin Oncol 2012;30: 1777–83.
- Lee DJ, Pajak TF, Stetz J, Order SE, Weissberg JB, Fischer JJ. A phase I/II study of the hypoxic cell sensitizer misonidazole as an adjunct to high fractional dose radiotherapy in patients with unresectable squamous cell carcinoma of the head and neck: A RTOG randomized study (#79-04). Int J Radiat Oncol Biol Phys 1989;16:465–70.
- Mizoe JE, Hasegawa A, Jingu K, Takagi R, Bessyo H, Morikawa T, et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother Oncol 2012;103:32–7.
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol 2011;100: 22–32.
- Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol 1996;6:10–21.
- Bassler N, Toftegaard J, Luhr A, Sorensen BS, Scifoni E, Kramer M, et al. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol 2014;53: 25–32.
- Thorwarth D, Alber M. Implementation of hypoxia imaging into treatment planning and delivery. Radiother Oncol 2010;97:172–5.
- Bentzen SM, Gregoire V. Molecular imaging-based dose painting: A novel paradigm for radiation therapy prescription. Semin Radiat Oncol 2011;21:101–10.
- Geets X, Gregoire V, Lee JA. Implementation of hypoxia PET imaging in radiation therapy planning. Q J Nucl Med Mol Imaging 2013;57:271–82.
- Grau C, Olsen DR, Overgaard J, Hoyer M, Lindegaard JC, Muren LP. Biology-guided adaptive radiation therapy – presence or future? Acta Oncol 2010;49:884–7.
- Grau C, Hoyer M, Alber M, Overgaard J, Lindegaard JC, Muren LP. Biology-guided adaptive radiotherapy (BiGART) – more than a vision? Acta Oncol 2013;52:1243–7.
- Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012;9:674–87.
- Carlin S, Zhang H, Reese M, Ramos NN, Chen Q, Ricketts SA. A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J Nucl Med 2014;55:515–21.
- Carlin S, Humm JL. PET of hypoxia: Current and future perspectives. J Nucl Med 2012;53:1171–4.
- Busk M, Mortensen LS, Nordsmark M, Overgaard J, Jakobsen S, Hansen KV, et al. PET hypoxia imaging with FAZA: Reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur J Nucl Med Mol Imaging 2013;40:186–97.
- Grosu AL, Souvatzoglou M, Roper B, Dobritz M, Wiedenmann N, Jacob V, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69:541–51.
- Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother Oncol 2012;105:14–20.
- Souvatzoglou M, Grosu AL, Roper B, Krause BJ, Beck R, Reischl G, et al. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: A pilot study. Eur J Nucl Med Mol Imaging 2007;34:1566–75.
- Trinkaus ME, Blum R, Rischin D, Callahan J, Bressel M, Segard T, et al. Imaging of hypoxia with 18F-FAZA PET in patients with locally advanced non-small cell lung cancer treated with definitive chemoradiotherapy. J Med Imaging Radiat Oncol 2013;57:475–81.
- Zips D, Zophel K, Abolmaali N, Perrin R, Abramyuk A, Haase R, et al. Exploratory prospective trial of hypoxia- specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol 2012;105:21–8.
- Servagi-Vernat S, Differding S, Hanin FX, Labar D, Bol A, Lee JA, et al. A prospective clinical study of F-FAZA PET-CT hypoxia imaging in head and neck squamous cell carcinoma before and during radiation therapy. Eur J Nucl Med Mol Imaging 2014;41:1544–52.
- Delouya G, Igidbashian L, Houle A, Belair M, Boucher L, Cohade C, et al. (1)(8)F-FDG-PET imaging in radiotherapy tumor volume delineation in treatment of head and neck cancer. Radiother Oncol 2011;101:362–8.
- Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K, Rajendran J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: Potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol 2011;101:369–75.
- Thorwarth D, Eschmann SM, Paulsen F, Alber M. Hypoxia dose painting by numbers: A planning study. Int J Radiat Oncol Biol Phys 2007;68:291–300.
- Bittner MI, Grosu AL. Hypoxia in head and neck tumors: Characteristics and development during therapy. Front Oncol 2013;3:223.
- Bittner MI, Wiedenmann N, Bucher S, Hentschel M, Mix M, Weber WA, et al. Exploratory geographical analysis of hypoxic subvolumes using F-MISO-PET imaging in patients with head and neck cancer in the course of primary chemoradiotherapy. Radiother Oncol 2013;108:511–6.
- Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999; 91:2081–6.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–78.
- Gregoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000;56:135–50.
- Gregoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol 2006;79: 15–20.
- Sterpin E, Differding S, Janssens G, Geets X, Gregoire V, Lee JA. Generation of prescriptions robust against geometric uncertainties in dose painting by numbers. Acta Oncol Epub 2014 July 3.
- Deveau MA, Bowen SR, Westerly DC, Jeraj R. Feasibility and sensitivity study of helical tomotherapy for dose painting plans. Acta Oncol 2010;49:991–6.
- Gregoire V MR, editors. ICRU report 83, International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J ICRU 2010;10(1).
- Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2001;49: 1171–82.
- Madani I, Duprez F, Boterberg T, Van de Wiele C, Bonte K, Deron P, et al. Maximum tolerated dose in a phase I trial on adaptive dose painting by numbers for head and neck cancer. Radiother Oncol 2011;101:351–5.
- Duprez F, De Neve W, De Gersem W, Coghe M, Madani I. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011;80:1045–55.
- Tran LB, Bol A, Labar D, Jordan B, Magat J, Mignion L, et al. Hypoxia imaging with the nitroimidazole 18F-FAZA PET tracer: A comparison with OxyLite, EPR oximetry and 19F-MRI relaxometry. Radiother Oncol 2012; 105:29–35.
- Sovik A, Malinen E, Skogmo HK, Bentzen SM, Bruland OS, Olsen DR. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. Int J Radiat Oncol Biol Phys 2007;68:1496–504.
- Berwouts D, Olteanu LA, Duprez F, Vercauteren T, De Gersem W, De Neve W, et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: Initial results of the phase I clinical trial. Radiother Oncol 2013; 107:310–6.
- van Kranen S, Mencarelli A, van Beek S, Rasch C, van Herk M, Sonke JJ. Adaptive radiotherapy with an average anatomy model: Evaluation and quantification of residual deformations in head and neck cancer patients. Radiother Oncol 2013;109:463–8.
- Robbins KT, Medina JE, Wolfe GT, Levine PA, Sessions RB, Pruet CW. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg 1991;117:601–5.
- Sobin L, Gospodarowicz M, Wittekind C, editor. TNM classification of malignant tumours, 7th ed. New York, NY, John Wiley and Sons, 2009.
