Abstract
Purpose/Objective. A phase II clinical trial evaluating the feasibility and outcome of treating locally advanced head and neck squamous cell carcinoma (HNSCC) with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin.
Material and methods. A total of 227 patients with stage III or IV HNSCC of the larynx, oropharynx, hypopharynx, or oral cavity where included between January 2007 and December 2010. The prescribed radiotherapy (RT) dose was 66–68 Gy in 2 Gy fractions, 6 F/W. The hypoxic radiosensitiser nimorazole was given orally at a dose of 1200 mg/m2 before each fraction. Concomitant cisplatin (40 mg/m2) i.v. was given once a week for a maximum of six cycles. Outcome data were evaluated in terms of loco-regional tumour control (LRC), event-free survival (EFS) and overall survival (OS). Morbidity data were evaluated based on the DAHANCA routine registration. Human papillomavirus (HPV)-status was estimated by immunohistochemical staining of p16.
Results. Included were 178 (78%) men and 49 (22%) women with a median age of 57 years. All except five patients received RT as prescribed. At least five series of cisplatin was given to 164 (72%) of the patients, and 149 patients (66%) received the full dose of nimorazole. The five-year actuarial LRC, EFS and OS rates were 80%, 67% and 72%, respectively. The LRC rates according to site were: oropharynx: 88%, larynx: 77%, hypopharynx 72% and oral cavity 49%, respectively. HPV/p16 staining was obtained in 141 of the 150 oropharyngeal cancers. Of these, 112 (79%) were p16 pos and 29 (21%) were p16 neg. LRC for the p16 neg oropharyngeal cancers was poorer than for the p16 pos (74% vs. 91%; p = 0.02). Tube feeding during treatment was necessary for 146 (64%) patients. At 12 months this number was reduced to 6%.
Conclusion. The treatment was tolerable in this cohort of locally advanced HNSCC patients. Acute and late toxicity was comparable to similar studies of chemoradiotherapy, and the outcome superior to the data reported in the literature. This strongly indicates that RT of advanced head and neck cancer must include as well hypoxic modification, accelerated fractionation as chemoradiotherapy to yield optimal outcome.
Radiotherapy (RT) is the main modality for treating locally advanced squamous cell carcinoma of the head and neck (HNSCC), and during the recent decades, several biological modifications have been introduced in order to improve the outcome. This includes modification of hypoxic radioresistance, attempts to overcome treatment-induced repopulation by accelerated fractionation and avoiding intrinsic radioresistance by combining RT with chemotherapy, preferably cisplatin. All three principles have subsequently been shown in large meta-analyses to be of substantial benefit [Citation1–5].
The DAHANCA group did initially through a number of large randomised trials explore the use of hypoxic modification and later followed by accelerated fractionation [Citation6–11]. Both principles were found to be beneficial and subsequently introduced into standard practice. Although of potential benefit [Citation12] the DAHANCA group was initially reluctant towards adding concomitant chemoradiotherapy, due to the risk of treatment limiting toxicity [Citation13,Citation14]. However, based on accumulating evidence of improved outcome, especially in advanced tumours with large nodal burden [Citation3,Citation4,Citation15] did we feel a need to add such principle to our treatment armamentarium. The past experience with concomitant chemoradiotherapy was mainly based on cisplatin combined with conventional fractionation [Citation3,Citation4], and it remained unclear to which extent such chemoradiotherapy could be given together with accelerated fractionation and hypoxic modification with nimorazole, without exceeding acceptable toxicity. The original concomitant cisplatin-based schedules were based on 100 mg/m2 every three weeks [Citation15], but the experience from regimes given to patients with uterine cervical cancer as well as some smaller studies in head and cancer, suggested that a weekly schedule with 40 mg/m2 may be more acceptable [Citation16,Citation17], and further that a total dose of 200 mg/m2 would be sufficient [Citation4,Citation18].
On this basis, we initiated a phase II trial to evaluate the effect and feasibility of such a schedule in the radiotherapeutic treatment of patients with locally advanced head and neck cancer.
The aim of the DAHANCA 18 protocol was to evaluate the loco-regional control (LRC) probability after RT with concomitant cisplatin with 40 mg/m2 weekly given with accelerated fractionation with 6 fractions per week of 2 Gy together with hypoxic modification with nimorazole 1200 mg/m2 given 90 minutes prior to RT [Citation19].
The phase II study was initiated in January 2007 and initially aimed at including 100 patients, but due to the increasing awareness of the change in patient characteristics due to human papillomavirus (HPV) positivity in most tumours, it was extended until the end of December 2010, ultimately recruiting 227 patients. A parallel protocol (DAHANCA 14) did evaluate the same treatment principle in patients with nasopharyngeal carcinoma.
Material and methods
Patients
Patients were eligible if they had histological confirmed stage III or IV (UICC 2002) squamous cell carcinoma of the laryngeal-, oropharyngeal-, hypopharyngeal- or oral mucosa and sufficient renal function (Cr-EDTA ≥ 50 ml/min). Between January 2007 and December 2010 a total of 227 patients were included from four Danish centers (Herlev, Odense, Aarhus and Aalborg). Patient and tumour characteristics were as described in . The median follow-up time for the survivors was 55 months.
Table I. Patient and tumour characteristics.
Treatment
Patients were treated according to the DAHANCA RT guidelines (http://www.dahanca.dk) and the DAHANCA, EORTC, EORTEC, NCIC, RTOG consensus guidelines from 2003 [Citation20]. The prescribed dose was 66–68 Gy in 2 Gy fractions, 6 fractions per week (66 Gy if tumour is < 4 cm and 68 Gy if tumour > 4 cm). All patients were treated with 3DCT-based conformal RT. During the recruitment period IMRT was introduced to all centers, why the majority of patients were treated with this modality. Additional hypoxic modification with nimorazole 1200 mg/m2, 90 minutes before each fraction was standard. Concomitant cisplatin (40 mg/m2, maximum dose 70) i.v. was given once a week during RT for a maximum of six cycles.
Statistical and ethical considerations
All data were collected at the local centers and validated and processed by the DAHANCA data centre. The primary endpoint was five years LRC after RT. Secondary endpoints included local control at the T-position, regional control in the N-position, distant metastasis, event-free survival (EFS), overall survival (OS), acute and late treatment-related morbidity and compliance to treatment. The endpoint for LRC was recurrence in T- or N-site. The endpoint for OS was death of any cause and the endpoint used for EFS was any recurrence or relapse and or death [Citation21]. All tumour control and survival endpoints were measured from the first visit at the department of radiotherapy.
As the primary measure of toxicity we used the absence or presence of a feeding tube at specified times during and after treatment since it reflects the burden of treatment. In addition specific early and late radiation-related morbidity endpoint was scored. Statistical analyses were performed with STATA 10.
The study was performed according to the WHO Helsinki Declaration and approved by the relevant ethical and data protection committees (journal no. 2005-41-4802).
Results
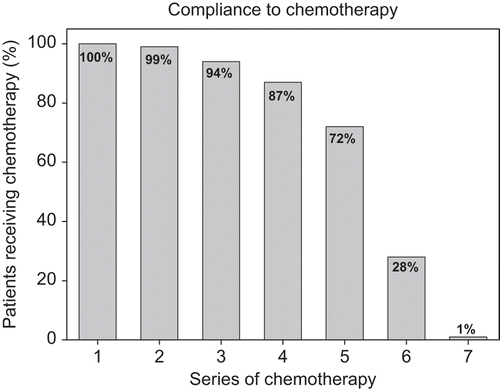
All but five patients received the prescribed total dose of RT. Four patients received 60 Gy and one patient 64 Gy. One hundred and sixty-four patients (72%) received five cycles of cisplatin or more (> 200 mg/m2) as shown in . Nimorazole was considered well tolerated by 149 patients (66%) who received the planned dose without reduction. The remaining 78 patients (34%) were registered with a reduced dose. This reduction was modest in 21 of the patients (9%), whom received in between 25 and 30 of the prescribed 30 daily doses. The major cause of dose reduction was due to nausea [Citation19].
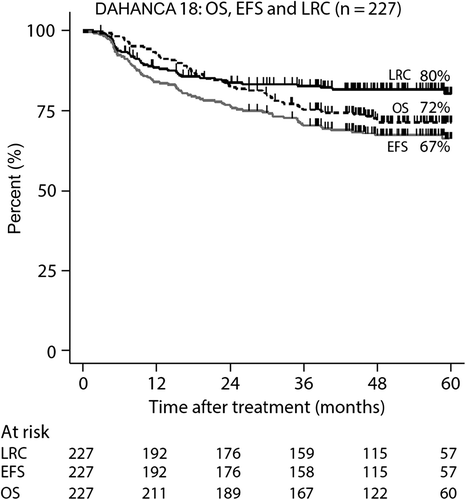
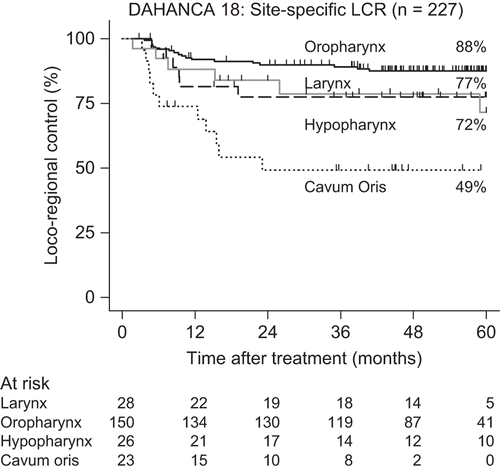
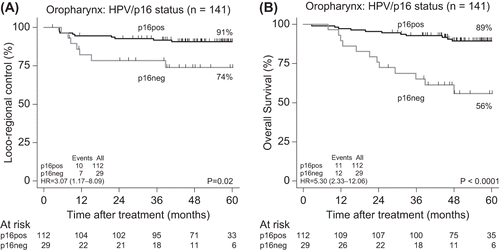
At the time of analysis 62 patients had died and 42 patients had experienced a loco-regional failure. Distant metastases were observed in 19 patients, of which eight were associated with loco-regional failure. The five-year actuarial rates of LRC, EFS and OS rates were 80% (CI 74–85%), 67% (CI 60–74%) and 72% (CI 66–78%), respectively (). The five-year probability of developing distant metastasis was 10% (Cl 8–12%). The LRC rates according to site of cancer where: larynx: 77% (CI 56–89%), oropharynx: 88% (CI 81–92%), hypopharynx 72% (CI 46–87%) and for the oral cancers: 49% (CI 27–68%) (). Site-specific OS were: larynx 64% (CI 44–79%), oropharynx 82% (CI 74–87%), hypophaynx 50% (CI 30–67%) and oral cavity 41% (CI 18–64%). Using EFS as endpoint was the site-specific five-year actuarial values: larynx 64% (CI 44–79%), oropharynx 77% (CI 69–83%), hypophaynx 45% (CI 26–63%) and oral cavity 35% (CI 17–54%). The five-year LRC rates for the 141 patients with oropharyngeal cancer according to p16 status were: p16 pos: 91% versus p16 neg: 74%, hazard ratio (HR) = 3.07 (1.17–8.09) (). OS rates for for the same groups were: p16 pos: 89% versus p16 neg: 56%, HR = 5.30 (2.33–12.06) (). Among the 178 patients with known p16 status, the proportion of T1-T2 tumours was significantly larger among the p16 pos tumours (79%) than among the p16 neg tumours (41%), p < 0.001 ().
Toxicity
The treatment-related morbidity is presented in . A total of 201/227 (89%) patients experienced severe acute toxicity during the course of chemoradiotherapy, mainly being confluent mucositis in 173 (76%) patients and/or acute dysphagia in 168 (74%). Tube feeding during treatment was necessary for 146 (64%) patients. Eighty-two patients had a PEG tube and 64 had nasogastric tubes. At two months after end of treatment 82 (36%) patients were in need of a feeding tube. After 5–8 months this number was reduced to 23 (10%) and after one year further reduced to 11 (6%). Among the 181 patients followed for two years only six patients (3%) were still in need of a tube. Dysphagia was a prominent late effect and 19% had persistent dysphagia at two years after treatment ().
Table II. Acute and late radiation-related morbidity.
Discussion
The primary endpoint for this study was the LRC rate which was 80%. This result compares favourably to historical DAHANCA data from the combined DAHANCA 6 and 7 studies where patients treated with the accelerated RT had a five-year LRC of 70%. However, a direct comparison between the DAHANCA 18 and the DAHANCA 6 and 7 studies is not possible since the eligibility criteria were different and DAHANCA 6 and 7 also included patients with smaller stage and/or nasopharyngeal tumours. The five-year estimated OS of 72% in the DAHANCA 18 study is definitely a remarkably good result compared to the MACH-NC data, where the concomitant arm had a five-year survival of 34% [Citation4]. However, in the current study 150 of the 227 patients (66%) had oropharyngeal cancers, and of these 112 (75%) were p16 pos. The LRC and OS for the p16 pos patients were 91% and 90%, which is very high but reflect findings from other recent studies [Citation8,Citation18,Citation22]. The high proportion of p16 pos oropharyngeal cancers in our study population can undoubtedly explain a major part of the very favorable overall result as well as the high proportion of T1-T2, N-positive tumours.
However patients with laryngeal- and hypopharyngeal cancers did rather well too. The respective OS rates for larynx (64%) and hypopharynx (50%) are superior compared to the MACH-NC-site-specific study [Citation5] in which the same values were 47% and 30%, respectively. The OS for the oral cancers in the presented study (41%) is remarkably lower than for the laryngeal and hypopharyngeal cancers, but still superior to the result found in the MACH-NC (36%). Furthermore, the group of oral cancers in our study is somewhat unfavorably selected since the standard treatment for oral cancers according to DAHANCA guidelines is surgery if technically feasible. Thus, the oral cancer patients in the present study were those that were considered initially non-operable.
Most studies of chemoradiotherapy in advanced HNSCC have be performed with conventional fractionated RT [Citation4,Citation5]. However, the few recent, but large studies applying accelerated fractionation seems to cause an improve outcome [Citation18,Citation23,Citation24], although an increased amount of patients, with p16/HPV positive oropharyngeal tumours in these trials may also add to the better treatment results [Citation18,Citation24]. In the recent RTOG 0129 trial [Citation18] this was achieved with a reduced dose of cisplatin (200 mg/m2). Yet none of the previous studies which have been using accelerated fractionation have achieved at outcome similar to DAHANCA 18 irrespective of the p16/HPV status.
We did not have smoking data on all patients in the present cohort, but such information is mainly likely to influence the outcome in the HPV positive patient cohort. Among the non-HPV positive patients, the five-year rate of LRC and the OS were 72% and 57%, respectively. When excluding the patients with oral cavity tumours, the same values for the non-HPV positive larynx and pharynx stage III and IV patients were 73% and 54%, respectively. Such outcome is superior to what is found in the literature for similar patients treated with chemoradiotherapy [Citation18,Citation23], and is probably attributed to the combined therapy with accelerated fractionation, concomitant chemotherapy and not least hypoxic modification. Thus, our study strongly indicates that chemoradiotherapy in advanced HPV negative larynx and pharynx tumours may be further enhanced by addition of hypoxic modification. This question is currently being addressed in a large international randomised trial (EORTC 1219/DAHANCA 29) where the treatment regime in the DAHANCA 18 study is similar to the experimental arm of that randomised trial, which addresses the role of nimorazole in HPV negative tumours. It is noteworthy that the three-year LRC rate in our cohort of HPV negative larynx and pharynx tumours of 74% is of a similar magnitude as the expected outcome of the EORTC trial.
The only other study of hypoxic modification and chemoradiotherapy in patients with advanced HNSCC comparable to DAHANCA 18 is the TROG 0202 study where the potential effect of the hypoxiccytotoxin tirapazamin was evaluated in patients with advanced HNSCC also treated with cisplatin and conventional fractionated RT [Citation25]. This trial suffered from problems with RT compliance [Citation26], but analysis of the patients correctly treated gave a two-year LRC rate (75%) which was inferior to the comparable value of 83% observed in DAHANCA 18 study.
Our tumour control results are rather impressive, but may be further enhanced by adding a EGFr-inhibitor. It is unclear to which extent such additional treatment may yield benefit to patients also receiving concomitant chemotherapy or may be able to substitute cisplatin. The recent completed DAHANCA 19 trial used the present treatment schedule as control arm and added in the experimental arm the EGFr-antibody zalutumumab [Citation27]. However no additional improvement was found. Similar findings were observed in the RTOG 0522 study where addition of cetuximab to accelerated chemoradiotherapy was of no benefit [Citation24]. Currently, therefore the present treatment schedule with accelerated RT, hypoxic modification and weekly cisplatin seems to be the most suitable treatment to advanced HNSCC, irrespective of p16/HPV status.
Compliance and toxicity
Compliance to the treatment schedule was rather good as 72% of the patients received at least five series of cisplatin and all except five patients received RT as per schedule. The received dose of the radiosensitiser nimorazole was reduced in 34% cases and most often terminated due to nausea [Citation19].
The acute and late morbidity was acceptable and in the same order as that found in other studies [ 14,18,23,24,28,29]. This indicates that the combination of weekly cisplatin with accelerated fractionation and nimorazole was feasible and that such treatment can be applied into a cohort of patients with advanced head and neck carcinoma. Similar, the compliance is at the same level of what is found in the other studies of cisplatin-based chemoradiotherapy but without acceleration or hypoxic modification.
Tube feeding was necessary for 64% of the patients during treatment. This reflects the attenuation of the acute toxicity, when cisplatin is combined with RT, as also described in several previous studies with concomitant chemotherapy [Citation14,Citation23,Citation28,Citation29]. Most of the patients treated with tube feeding in our study population, however, managed without the tube already few months after the end of treatment. Only 6% needed tube feeding after one year, and 3% after two years. This is somewhat lower than reported in most other studies [Citation30] and may be due to a reduced extent of late toxicity with our schedule (66–68 Gy total vs. 70 Gy and cisplatin 40 mg/m2 vs. 100 mg/m2) but could also be due to differences in the efforts taken to train the patients to manage without their tubes.
Conclusion
In the DAHANCA 18 study weekly cisplatin was added to a moderately accelerated RT regimen together with hypoxic modification. The treatment was tolerable in this cohort of locally advanced head and neck cancer patients. Acute toxicity demanded tube feeding for a major part of the patients during treatment, but only 3% were in need of the tube two years after treatment. The loco-regional and survival rates for the entire group of patients in this study (OS: 72%, EFS: 67% and LRC: 80%) were remarkably good compared to contemporary studies. Some of this improvement must be attributed to the fact that almost half of the patients had p16/HPV positive oropharyngeal tumours. However, patients with cancer at other sites also had a superior outcome when compared to recent literature. The future challenge (and trials) should be targeted towards identify the patients in need of such treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol 2011;100:22–32.
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Meta-analysis of Radiotherapy in Carcinomas of Head and Neck (MARCH) Collaborative Group. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006;368:843–54.
- Pignon JP, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000;355:949–55.
- Pignon JP, le Maître A, Maillard E, Bourhis J, MACH-NC Collaborative Group.Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14.
- Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother Oncol 2011;100:33–40.
- Overgaard J, Hansen HS, Andersen AP, Hjelm-Hansen M, Jørgensen K, Sandberg E, et al. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: Report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys 1989;16:1065–8.
- Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85 .Radiother Oncol 1998;46:135–46.
- Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009;27:1992–8.
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003;362:933–40.
- Mortensen HR, Overgaard J, Specht L, Overgaard M, Johansen J, Evensen JF, et al. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6 & 7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother Oncol 2012;103:69–75.
- Mortensen HR, Overgaard J, Jensen K, Specht L, Overgaard M, Johansen J, et al. Factors associated with acute and late dysphagia in the DAHANCA 6 & 7 randomized trial with accelerated radiotherapy for head and neck cancer. Acta Oncol 2013;52:1535–42.
- Overgaard J, Khan AR. Selective enhancement of radiation response in a C3H mammary carcinoma by cisplatin. Cancer Treat Rep 1981;65:501–3.
- Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol 2007;25:4096–103.
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 2008;26:3582–9.
- Adelstein DJ, Li Y, Adams GL, Wagner H, Jr., Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–8.
- Thomas GM. Improved treatment for cervical cancer – concurrent chemotherapy and radiotherapy. N Engl J Med 1999;340:1198–200.
- Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in loco regionally advanced nasopharyngeal carcinoma: Progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002;20: 2038–44.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35.
- Metwally MA, Frederiksen KD, Overgaard J. Compliance and toxicity of the hypoxic radiosensitizer nimorazole in the treatment of patients with head and neck squamous cell carcinoma (HNSCC). Acta Oncol 2014;53:654–61.
- Grégoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol 2003;69:227–36.
- Michiels S, Le Maître A, Buyse M, Burzykowski T, Maillard E, Bogaerts J, et al. MARCH and MACH-NC Collaborative Groups. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol 2009;10:341–50.
- Lassen P. The role of human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol 2010;95:371–80.
- Bourhis J, Sire C, Graff P, Grégoire V, Maingon P, Calais G, Gery B, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol 2012;13:145–53.
- Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014;32:2940–50.
- Rischin D, Peters LJ, O’Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): A phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010;28:2989–95.
- Peters LJ, O’Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: Results from TROG 02.02. J Clin Oncol 2010;28:2996–3001.
- Eriksen JG, Maare C, Johansen J, Primdahl H, Evensen JF, Kristensen CA, et al.DAHANCA19: A randomized phase III study of primary (chemo-) radiotherapy and zalutumumab in head and neck carcinomas. Radiother Oncol 2014;111(Suppl 1):154–5.
- Garden AS, Harris J, Trotti A, Jones CU, Carrascosa L, Cheng JD, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: A phase II trial of the radiation therapy oncology group (RTOG 99-14). Int J Radiat Oncol Biol Phys 2008;71:1351–5.
- Machtay M, Moughan J, Farach A, Martin-O’Meara E, Galvin J, Garden AS, et al. Hypopharyngeal dose is associated with severe late toxicity in locally advanced head-and-neck cancer: An RTOG analysis. Int J Radiat Oncol Biol Phys 2012;84:983–9.
- Koyfman SA, Adelstein DJ. Enteral feeding tubes in patients undergoing definitive chemoradiation therapy for head-and-neck cancer: A critical review. Int J Radiat Oncol Biol Phys 2012;84:581–9.