Abstract
Objective. This study aims to assess the effect of voice rehabilitation on health-related quality of life (HRQL) and communication experience for laryngeal cancer patients treated with radiotherapy.
Method. This prospective randomised controlled trial included 74 patients with Tis-T4 laryngeal cancer treated curatively by radiotherapy, of which 37 constituted the intervention group receiving voice rehabilitation and 37 patients as a control group. Patients were followed at one and six months post-radiotherapy, with voice rehabilitation conducted between these time-points. Endpoints included patient reported outcomes, including HRQL as measured by European Organisation for Research and Treatment of Cancer (EORTC) Core30 (C30) and Head & Neck35 (H&N35) as well as communication function as measured by Swedish Self-Evaluation of Communication Experiences after Laryngeal cancer (S-SECEL).
Results. The intervention group reported statistically significant improvements in communication experience as measured by S-SECEL environmental, attitudinal and total score domains compared to the control group. Similar improvements were seen in EORTC H&N35 Speech domain and the EORTC C30 domain Global quality of life. Moderate correlations were noted (r = 0.51–0.59) between three of four S-SECEL domains and the EORTC domains Speech and Global quality of life.
Conclusion. Laryngeal cancer patients treated with radiotherapy who receive voice rehabilitation appear to experience beneficial effects on communication function and selected HRQL domains. Voice rehabilitation following radiotherapy is recommended but further research investigating potential target groups and long-term effects is required.
Radiotherapy following laryngeal cancer can affect voice function, both as assessed by trained professionals as well as reported by the patients themselves [Citation1–3]. Longitudinal multidimensional studies demonstrate that 12 months following radiotherapy completion, patients either improve or report findings similar to pre-treatment values, but still remain abnormal [Citation1,Citation4]. These voice problems persist to some extent for as long as 10 years after radiotherapy [Citation5,Citation6].
Voice and communicative ability have proved particularly important for this patient population and suboptimal function has been shown to negatively influence social activities and health-related quality of life (HRQL) [Citation3,Citation7]. Thus, the potential benefits of offering voice rehabilitation have been discussed but to the best of our knowledge only three studies have investigated this in radiotherapy-treated laryngeal cancer patients, all indicating positive effects but with hampering limitations [Citation8–10], albeit none have investigated correlations between voice rehabilitation, communication function and HRQL. Fex et al. concluded that voice training given concomitant with radiotherapy was effective for their 15 patients. However, the study had few patients, no control group was employed and it is unclear what variable was used to measure improvement. Van Gogh et al, however, did utilise a control group but still only included 23 patients where the time from treatment (radiotherapy or laser surgery) to voice rehabilitation ranged from 6 to 120 months. Similarly, the randomised study by Tuomi et al. also had a small cohort of 20.
It may be concluded that convincing evidence for the efficacy of voice rehabilitation in patients treated with radiotherapy for laryngeal cancer is lacking. Hence, the main objective of this randomised controlled study is to assess the effect of voice therapy on perceived communication and HRQL.
Method
Study participants
Patients diagnosed with primary laryngeal cancer in the western part of Sweden between the years 2000 and 2011, who were to receive curatively intended radiotherapy, were asked to participate in the study. Inclusion criteria were sufficient cognitive abilities and Swedish language competency in order to independently fill in the questionnaires and be able to participate in voice rehabilitation sessions. Comorbidity was measured using the Adult Comorbidity Evaluation (ACE-27) [Citation11,Citation12].
Study design
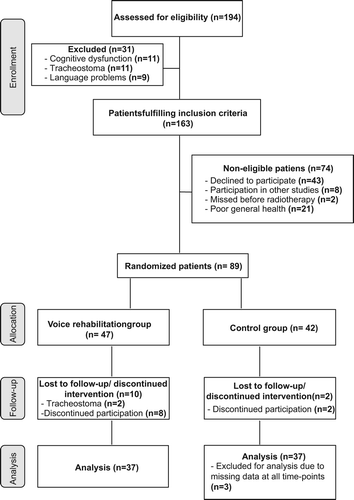
The study design consisted of a randomised controlled study encompassing assessment of voice function and patient-reported outcome (PRO) at baseline (one month post-radiotherapy) and six months post-radiotherapy. Computerised randomisation was performed by optimal allocation using Pocock's sequential randomisation method [Citation13] applied to age, smoking habits, tumour site, size and patient's self-evaluation of communication as assessed by S-SECEL pre-radiotherapy. Sample size was determined by 80% power calculation with dysphonia as defined by Stoicheff et al. [Citation14] as the main variable. A total of 163 patients fulfilled the study inclusion criteria, of which 74 declined participation (). A total of 89 patients were available for randomisation into two groups: an intervention group receiving voice rehabilitation or a control group. However, during the intervention period, 12 patients discontinued participation. A further three were excluded from analysis due to missing data for both study time-points, leaving 74 patients available for analysis.
Oncologic treatment
During the study period, traditional radiotherapy treatment was administered. Oncologic treatment was given as conventional (n = 52) or hyperfractionated (n = 22) radiotherapy according to regional guidelines. The former was administered as 34/26 fractions of 2–2.4 Gy fractions daily totalling 62.4–68 Gy. The latter regimen encompassed 1.7 Gy fractions given twice daily, resulting in 38 fractions and a total of 64.6 Gy. Lymph nodes, levels II–IV, were irradiated in all patients with sub and supraglottic tumours as well as those with T2 or larger glottic tumours (n = 28) totalling 40.8 Gy in the hyperfractionated group (n = 19) and 46 Gy in the conventionally fractionation group (n = 9). T3 and T4-tumours also received induction chemotherapy (n = 3) unless contraindicated.
Intervention: Voice rehabilitation
Voice rehabilitation was conducted in line with a structured protocol at the hospital in closest proximity to the patient's residence. It commenced approximately one month following radiotherapy completion and was given by trained speech-language pathologists in the research group. The protocol was developed according to Swedish standard voice training prior to the study starting. Voice rehabilitation consisted of 10 × 30 minute sessions over 10 weeks and included relaxation, respiration, posture and phonation exercises [Citation15]. The patients were asked to conduct voice training daily at home in between sessions. The control group did not receive any voice rehabilitation but were followed with recordings and PRO instruments in parallel with the study group. The control group received vocal hygiene advice, according to standard practice.
European Organisation for Research and Treatment of Cancer
The European Organisation for Research and Treatment of Cancer (EORTC) Quality-of-Life Questionnaire Core-30 (QLQ-C30) is a questionnaire developed to evaluate HRQL for the general cancer population. The QLQ-C30 consists of 30 items focusing on physical and psychosocial functioning as well as symptoms that cancer patients experience. A disease-specific head and neck (H&N) module has been developed to complement this (EORTC QLQ-H&N35) and consists of 35 items addressing symptoms specific to H&N cancer and its treatments. Calculated scores range from zero to 100. Higher scores for the Functional domain and Global quality of life (QOL) represent better function, whereas higher scores for symptom domains and single-items indicate a higher symptom burden. A change in score of ≥ 10 points could be considered clinically relevant[Citation16,Citation17] and is frequently used when interpreting results in the H&N35 as well.
Swedish Self-Evaluation of Communication Experiences after Laryngeal Cancer
The Swedish version of the Self-Evaluation of Communication Experiences after Laryngeal cancer (S-SECEL) is a 35-item questionnaire adapted to assess communicative function for patients treated for laryngeal cancer. The instrument has been found reliable, sensitive and valid [Citation6,Citation18,Citation19]. The 35 items are divided into three domains; General, Environmental and Attitudinal, as well as a single-item scale. The General domain assesses general attitudes about being relaxed and calm as well as acknowledgement of the sickness and treatment. The Environmental domain focuses on efficiency of voice usage in different environments, such as in a large room, with a group of people etc. The Attitudinal domain addresses attitudes about speech. Items are rated on a four-point Likert scale ranging from never (0) to always (3). Domain summary scores are calculated for all three domains (General 0–15, Environmental 0–42, Attitudinal 0–45) as well as a Total score (0–102). A reduction of ≥ 2, ≥ 7, ≥ 4 or ≥ 13 points in the General, Environmental, Attitudinal and Total score domains, respectively, may represent a minimum clinically relevant improvement [Citation20]. The last question “Do you speak the same amount now as before your laryngeal cancer” is answered as Yes/More/Less and is not included in the scoring system.
Statistical analysis
For descriptive purposes data is presented using mean, standard deviation, 95% confidence interval (CI) for the mean and effect size (ES: Mean value six months post-radiotherapy – mean value one-month post-radiotherapy/standard deviation of the difference) for continuous variables and with number and percentage for categorical variables. ES was interpreted according to Cohen's standard criteria where size is classified as trivial (0–< 0.2), small (0.2–< 0.5), moderate (0.5–< 0.8) or large (≥ 0.8) [Citation21]. For comparisons between two groups Fisher's exact test was used for dichotomous variables, the χ2-test for non-ordered categorical data, the Mantel-Haenszel χ2 for ordered categorical variables and the Mann-Whitney U-test for continuous variables. For changes over time within groups Wilcoxon-signed test was used for continuous variables. In order to adjust for differences in baseline values between the two groups analysis of covariance (ANCOVA) was used. Spearman's correlation coefficient was used for all correlation analyses. All significance tests were two-sided and conducted at 5% significance level. The SPSS version 20.0 for Mac and SAS 9.3 was used for statistical analyses.
Ethical considerations
The study was conducted according to the Declaration of Helsinki and was approved by the Regional Ethic Review Board in Gothenburg, Sweden. All participants gave their informed consent before inclusion in the study. A part of this study population has been described previously [Citation15].
Results
Clinical characteristics of the control (n = 37) and intervention (n = 37) groups are shown in , and did not differ significantly between the groups. Analysis of the 89 patients that did not participate revealed statistically significant differences compared to the included patients in terms of site, age and smoking habit. The excluded patients were significantly older, more often non-smokers and had more subglottic and transglottic tumours.
Table I. Clinical characteristics of the intervention group and control group as well as of excluded patients.
EORTC QLQ-C30
Scores for EORTC QLQ-C30 and QLQ-H&N35 are presented in (complete data sets are presented in Supplementary Tables I and II, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.995773). Both groups showed trends of improvement between baseline and six months post-radiotherapy. The control group reported only small changes, apart from the Fatigue domain, which reached clinical significance (Δ10.8 p, ES = 0.52). The item Financial difficulties demonstrated a statistically significant improvement in the intervention group (p = 0.013, ES = 0.50). Overall, changes of greater magnitude were observed in the intervention group when compared to the control group, particularly for the domains Role functioning, Social functioning and Global QOL ranging from Δ 15 to 21 points (ES 0.89–1.7, p < 0.001). These were also statistically significant improvements when compared to the control group. After adjustment of magnitude of change due to differing inter-group scores at baseline, a clinically and statistically significant improvement remained for the domain Global QOL and the item Financial difficulties in the intervention group.
Table II. EORTC QLQ-C30 and QLQ-H&N35 scores presented as mean (SD) (95% CI), p-values and effects size (ES) for baseline and six months post-radiotherapy.
EORTC QLQ-H&N35
The intervention group reported more improvements, which were significantly better when compared to the control group especially in the domains Speech and Social contact following voice rehabilitation (six months post-radiotherapy). This was not seen in the control group. The differences were statistically significant (p < 0.001 and p = 0.005, respectively), where the improvement for the Speech domain reached clinical significance. Changes in the remaining H&N35 domains and items were mostly non-significant and of small magnitude.
S-SECEL
The S-SECEL scores are presented in . The control group scores remained static, whilst the intervention group reported scores indicative of changes of large magnitude (0.93–1.1) for all domains and clinically relevant changes, with the exception of S-SECEL General domain. Excluding the General domain, all inter-group differences were statistically significant even after adjustment for scores differing between groups at baseline.
Table III. S-SECEL scores presented as mean (SD) (95% CI), p-values and effect size (ES) at baseline and six months post-RT.
Correlation of change
Correlation of change between all S-SECEL domains and selected HRQL domains (EORTC C30 Social functioning, C30 Role functioning, C30 Global QOL, H&N35 Speech and H&N35 Social contact) are shown in . The S-SECEL General domain correlated weaker with all HRQL-domains and items compared to the other S-SECEL domains. Remaining S-SECEL domains correlated weaker with the domains Role functioning (r = 0.33–0.36) and Social functioning (r = 0.27–0.30) as well as the domain Social contact (r = 0.19–0.41). Stronger correlations (r = 0.51–0.59) were observed between S-SECEL Environment, Attitudinal and Total score domains and the domains Global QOL and Speech.
Table IV. Correlations of change between S-SECEL and selected EORTC C30 and H&N35 domains presented as r (p-value).
Discussion
This is the first prospective randomised controlled trial that investigates effects of voice rehabilitation on HRQL, perceived communication function and voice in laryngeal cancer patients treated with radiotherapy.
First, voice rehabilitation appears effective in terms of perceived communicative ability measured by S-SECEL as the intervention group reported clinically significant improvements on all domains except for the General domain. The improvements were statistically significantly greater when compared to the control group. Tuomi et al. [Citation15] found results in line with these in their 33 male laryngeal cancer patients also described in this study using only the S-SECEL Environmental domain. An improvement was also demonstrated by Van Gogh et al. [Citation8], where the 12 patients receiving voice therapy reported a 15-point improvement on the Voice Handicap Index (VHI) compared to three points in the control group. Nevertheless, the S-SECEL values reported in this study are still inferior when compared to those of healthy reference samples [Citation22].
These patient-reported communicative improvements are also mirrored in the HRQL domains Speech and Social contact, which was expected. The correlation between the Speech domain and S-SECEL implies that the instruments measure slightly different constructs. Thus, it emphasises the need for S-SECEL as an adjunct to the EORTC when highlighting communication experiences in this patient cohort, as the speech domain consists of only three voice-related items, which may not be sensitive enough to use on an individual level. This has previously been demonstrated by Johansson et al. [Citation19]. The intervention group also reported a significant improvement in the item Financial difficulties compared to the control group. Possibly, more professional voice users in this group could explain the results. However, in order to investigate this further more extensive health-economy analyses would be required and were out of the scope of this study. Furthermore, only weak correlations were seen between S-SECEL and the domains Role functioning, Social functioning and Social contact. This may be explained by S-SECEL focusing specifically on communicative ability whereas the EORTC domains encompass only 2–5 items each and cover limitations in the patient's work, daily activities, leisure or family life as a result of all aspects of the disease. Hence, communicative function might have little impact on the above-stated domains. If communicative function is the primary endpoint, S-SECEL should be the preferred instrument.
It has been documented that speech is particularly important for patients with early laryngeal cancer and that psychological distress and feelings of isolation result from lack of communication [Citation7,Citation23]. It was not surprising that the intervention group reported statistically and clinically significant improvements in the Global QOL domain, which were not observed in the control group. Three of four S-SECEL domains correlated moderately with the Global QOL domain, indicating that voice rehabilitation may impact the patient's overall QOL. Rinkel et al. recently demonstrated a similar moderate correlation albeit using the VHI in laryngeal cancer patients treated by radiotherapy [Citation24]. This study, however, is the first to document improvements in Global QOL following voice rehabilitation.
Nevertheless, improvements reported by the patients are not always reflected in other methods used for measuring voice function. This was noted by Van Gogh et al. [Citation8] where perceptual voice qualities of roughness and breathiness remained unchanged post-voice therapy, which highlights the issue of how treatment efficacy should be measured. Perceptual evaluation is a blinded opinion albeit of an expert or naïve listener, whilst PRO scores represent the patient's viewpoint and experience of their voice. Although perceptual rating is regarded as gold standard when assessing voice function [Citation25], we advocate that patients are followed using voice recordings on an individual level with the addition of PRO usage since an important assessment of patient satisfaction with treatment will be determined by the patients themselves. However, the structured voice rehabilitation employed in this study may not be optimal for all patients and speech-language pathologists are therefore vital in evaluating and tailoring voice rehabilitation for those in need.
This study is limited by several PRO measures differing at baseline between the intervention and control group (S-SECEL, EORTC QLQ-C30 Role functioning and H&N35 speech). Despite addressing this by adjusting the magnitude of inter-group change, significant findings were still observed. Additionally, patients in the excluded group were significantly older and of more subglottic and transglottic location, thereby hampering generalisation of the results.
Conclusion
Laryngeal cancer patients treated with radiotherapy who receive voice rehabilitation appear to experience beneficial effects on communication function and HRQL. Voice rehabilitation following radiotherapy is recommended but further research investigating potential target groups and long-term effects is warranted.
Supplementary material available online
Supplementary Table I–II to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.995773.
ionc_a_995773_sm9064.docx
Download MS Word (47.7 KB)Acknowledgements
This study was supported by the Swedish Cancer Society, Assar Gabrielsson Foundation, the Research and Development Council (FoU), The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Gothenburg University and the Sahlgrenska University Hospital Foundation. We would like to thank the dedicated speech-language pathologists in the Va stra Go taland County for helping in collecting the data and Carina A berg for her help in including the patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bibby JR, Cotton SM, Perry A, Corry JF. Voice outcomes after radiotherapy treatment for early glottic cancer: assessment using multidimensional tools. Head Neck 2008;30: 600–10.
- Jacobi I, van der Molen L, Huiskens H, van Rossum MA, Hilgers FJ. Voice and speech outcomes of chemoradiation for advanced head and neck cancer: A systematic review. Eur Arch Otorhinolaryngol 2010;267:1495–505.
- Peeters AJ, van Gogh CD, Goor KM, Verdonck-de Leeuw IM, Langendijk JA, Mahieu HF. Health status and voice outcome after treatment for T1a glottic carcinoma. Eur Arch Otorhinolaryngol 2004;261:534–40.
- Karlsson T, Bergström L, Ward L, Finizia C. A prospective longitudinal study of voice characteristics and quality-of-life outcomes following laryngeal cancer treatment with radiotherapy. Unpublished manuscript.
- Hocevar-Boltezar I, Zargi M, Strojan P. Risk factors for voice quality after radiotherapy for early glottic cancer. Radiother Oncol 2009;93:524–9.
- Finizia C, Bergman B, Lindstrom J. A cross-sectional validation study of self-evaluation of communication experiences after laryngeal cancer – a questionnaire for use in the voice rehabilitation of laryngeal cancer patients. Acta Oncol 1999;38:573–80.
- Metcalfe CW, Lowe D, Rogers SN. What patients consider important: Temporal variations by early and late stage oral, oropharyngeal and laryngeal subsites. J Craniomaxillofac Surg 2014;42:641–7.
- van Gogh CD, Verdonck-de Leeuw IM, Boon-Kamma BA, Rinkel RN, de Bruin MD, Langendijk JA, et al. The efficacy of voice therapy in patients after treatment for early glottic carcinoma. Cancer 2006;106:95–105.
- Fex S, Henriksson B. Phoniatric treatment combined with radiotherapy of laryngeal cancer for the avoidance of radiation damage. Acta Otolaryngol Suppl 1969;263:128–9.
- Tuomi L, Bjorkner E, Finizia C. Voice outcome in patients treated for laryngeal cancer: Efficacy of voice rehabilitation. J Voice 2014;28:62–8.
- Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. J Chronic Dis 1974;27:387–404.
- Piccirillo JF, Feinstein AR. Clinical Symptoms and Comorbidity: Significance for the Prognostic Classification of Cancer. Cancer 1996;77:834–842.
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15.
- Stoicheff ML, Ciampi A, Passi JE, Fredrickson JM. The irradiated larynx and voice: A perceptual study. J Speech Hear Res 1983;26:482–5.
- Tuomi L, Andrell P, Finizia C. Effects of voice rehabilitation after radiation therapy for laryngeal cancer: A randomized controlled study. Int J Radiat Oncol Biol Phys 2014;89: 964–72.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011; 29:89–96.
- Finizia C, Palme C, Bergman B. A longitudinal study of the Swedish Self-Evaluation of Communication Experiences after Laryngeal Cancer questionnaire in patients treated for laryngeal cancer. Acta Oncol 2002;41:262–8.
- Johansson M, Ryden A, Finizia C. Self evaluation of communication experiences after laryngeal cancer – a longitudinal questionnaire study in patients with laryngeal cancer. BMC Cancer 2008;8:80.
- Tuomi L, Johansson M, Andrell P, Finizia C. Interpretation of the Swedish Self Evaluation of Communication Experiences after Laryngeal cancer (S-SECEL): Cut-off levels and minimum clinically important differences. Head Neck 2014.
- Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Press; 1988.
- Tuomi L, Karlsson T, Johansson M, Finizia C. Health-related quality of life and voice following radiotherapy for laryngeal cancer – a comparison between glottic and supraglottic tumours. Acta Oncol Epub 2014 June 10.
- Happ MB, Roesch T, Kagan SH. Communication needs, methods, and perceived voice quality following head and neck surgery: A literature review. Cancer Nurs 2004; 27:1–9.
- Rinkel RN, Verdonck-de Leeuw IM, van den Brakel N, de Bree R, Eerenstein SE, Aaronson N, et al. Patient-reported symptom questionnaires in laryngeal cancer: Voice, speech and swallowing. Oral Oncol 2014;50:759–64.
- Bhuta T, Patrick L, Garnett JD. Perceptual evaluation of voice quality and its correlation with acoustic measurements. J Voice 2004;18:299–304.