Abstract
Background. Fatigue is a symptom that can occur during treatment as an acute side effect but can also result in persistent fatigue as a long-term side effect or late effect.
Materials and methods. We undertook a narrative review of the current literature and discuss the current evidence of assessment of fatigue and we specifically focus on the role of promoting behavioural change and focused rehabilitation to minimise these long-term effects and update the literature relating to this area from 2012 to date.
Results. We suggest there are behavioural change models that can be scaled up to enable patients to manage long-term fatigue using exercise. However, from this updated review there are limitations to the current infrastructure and evidence base that will impact on the ability to do this.
Conclusion. We continually need to raise awareness amongst health professionals to continue to suggest modifications to impact on fatigue at all stages of cancer treatment and into survivorship and late effects. These can range from simple brief interventions suggested in the clinic to full scale rehabilitation programmes if the correct infrastructure is available. Whichever approach is adopted we suggest exercise will be the mainstay of the treatment of fatigue in this group.
Chronic fatigue (debilitating tiredness impacting on function and lasting for potentially months) is now a well recognised phenomenon in people successfully treated for cancer [Citation1]. The majority of the evidence comes from breast cancer and as this is a subjective symptom there is a wide range of figures [Citation2]. Fatigue appears to affect about 30% of those treated although this rough figure belies its complex nature [Citation3]. The general trend is for fatigue to diminish over time – the majority improves without intervention following the end of cancer treatment. Although this may mean a small percentage are affected for up to five years following treatment [Citation4]. The growing absolute numbers of cancer survivors means there is the potential for economic and occupational impact and effects should fatigue persist even in the minority. There is also the additional increased use of healthcare resources as a secondary problem [Citation5]. We will address some of the reasons for the development of fatigue, and explore the optimum time and methods using exercise to intervene to minimise its effects and duration.
Mechanisms of fatigue
We will initially discuss some of the proposed reasons for chronic fatigue. Fatigue is prolonged and modified by a number of biological and psychosocial mediators, such as mood and sleep disturbance, pain, cardiac dysfunction and lack of exercise [Citation2,Citation6]. There is potentially a large variation between patients and in individuals over time. The mechanism that causes and propagates fatigue remains unclear [Citation6,Citation7]. There is certainly an inflammatory response to both the cancer itself and the range of treatment modalities. A clear correlation exists between increased fatigue and inflammatory cytokines. The response seems most marked from the current evidence with chemotherapy but radiotherapy also causes a similar set of symptoms [Citation8]. There are associated central symptoms, such as sleep and memory disturbances, that occur uniquely related to cancer treatment which overlaps with fatigue [Citation3,Citation9,Citation10]. This process is likely to be driven by cytokines that cross the blood brain barrier and can act centrally. The mechanism by which exercise can modify some or all of these effects is not clear and many may be non-specific [Citation11]. However, there is also evidence that cancer patients rapidly lose muscle mass either through inactivity or by this inflammatory process. There is potentially a role for further biomarker studies to provide further objective measures as to the exercise ‘dose response’ [Citation12]. Exercise will also have an impact on the associated psychological correlates of fatigue, such as mood and sleep disturbance. While these are not formal comorbidities they occur in parallel to fatigue and will have an identifiable impact [Citation13]. This review is not intended to examine the causes of fatigue in detail but examine the role exercise has on fatigue and its associated symptoms. A detailed examination of the postulated causes of fatigue can be found in recent reviews [Citation1,Citation6].
Medical comorbidities
There are also a large percentage of patients that have additional medical co-morbidities and so there are further indirect causes of fatigue although this may be mediated by cardiac dysfunction, endocrine or psychiatric causes [Citation14–17]. Fatigue is a recognised symptom in patients with cardiac dysfunction but this has not been routinely measured in the post-treatment cancer survivors [Citation18]. With an ageing population there is an increased likelihood of comorbidities [Citation19] and there will be an effect on the prevalence of fatigue because of this [Citation20].
What is clear from the growing number of epidemiological studies is that fatigue increases from the time of diagnosis throughout treatment [Citation21]. The rate and degree of change does vary between studies though and means the optimum timings of any intervention to improve fatigue remain unclear.
The general expert consensus would be to intervene during the early stages of treatment but this is often an overwhelming time for the patient and the focus is elsewhere [Citation22]. However, there is an increasing body of evidence that the period in and around the time of treatment patients want to become more active, engage in an activity they enjoy and have the tailored support to make this change [Citation23]. It is this area we will focus on as the suggestion is that reducing fatigue during treatment may help to reduce the incidence on fatigue in the post-treatment period [Citation23].
Updated evidence for exercise reducing fatigue from systematic reviews
Overall the evidence for exercise improving fatigue is increasing with a recently updated Cochrane review and other systematic reviews, e.g. [Citation11,Citation24,Citation25]. While the studies included in these reviews have predominately been in breast cancer there is a consistent positive overall effect on fatigue through exercise. The authors concluded from over 50 RCTs and over 1500 participants. Exercise was seen to be statistically more effective than the control intervention, e.g. in the Cochrane review [Citation24]: [standardised mean difference (SMD) −0.27, 95% confidence interval (CI) −0.37–−0.17; p = 0.005]. This is very similar in the other more recent reviews [Citation11,Citation25]. The majority of studies were post-treatment and conducted in breast and prostate cancer – all of which demonstrated benefit. However, those with haematological malignancies failed to benefit. The exact reason for this is unclear – it may be that more intensive treatment and possible greater immunosuppression had a more adverse effect on muscle mass and function. It is interesting to note aerobic exercise significantly reduced fatigue but resistance training and alternative forms of exercise failed to reach significance. This suggests that fatigue has a central cause and is not directly caused as a result of changes in muscle architecture [Citation6,Citation26]. The limitation of many of this type of study is that the focus is often on the overall level of physical functioning and fitness which does not correlate with the subjective symptoms of fatigue [Citation27].
We will therefore address when is the best time for these interventions to be introduced as the evidence is clear that fatigue can be addressed during treatment [Citation12,Citation28] and that analyses suggest there is a dose response too. While physical functioning is improved with earlier interventions there is no clear dose response with more intensive exercise and secondary symptoms, such as fatigue, are often recorded in insufficient detail to gauge the impact [Citation29]. Our stipulation is that the introduction of simple but repeated exercise interventions is likely to have the greatest impact on fatigue during treatment and potentially impact the prevalence of chronic fatigue as a result. We also need to include interventions that can be made even on intensive treatment and many research groups are adopting this approach [Citation30–33].
Timings of exercise interventions
We will now discuss when exercise should be started during cancer treatment. The updated guidelines and expert consensus, e.g. [Citation2] demonstrates that it is safe to be active during and after most types of treatment and the recommendation is that people gradually build back up to the age appropriate guidelines. For adults there are three recommendations firstly to reduce the amount of time spent sedentary, e.g. sitting and getting up and about at regular intervals; second aerobic exercise, 150 minutes (in 10 minute intervals) over the course of a week at a moderate intensity (enough to increase breathing rate and start to feel a little warmer but still be able to hold a conversation) and third – strength building activities. For older adults the recommendations also include two balance activities to help mitigate against falls and maintain independence [Citation2].
The benefits for fatigue start at a gentle level of physical activity, a recent clinical trial in chronic fatigue syndrome provides a good reference point on how to maximise the benefits that physical activity has to offer without patients exacerbating symptoms of fatigue [Citation34]. While it may not be possible to completely extrapolate directly to cancer patients, as we do know there are differences between these populations, but also an important number of similarities [Citation35] which can guide intervention design.
Behavioural change and exercise to minimise fatigue
While exercise has demonstrable benefits in reducing fatigue there still remains questions of how best to enable people to become and stay physically active, and sustainably build this into clinical care pathways across health care. The evidence on behavioural change to sustain this level of activity is limited [Citation25,Citation36].
If we examine the evidence in more detail there are useful illustrative trials and also pilot regional programmes that provide a more pragmatic structure to adopt these approaches into clinical practice. A tension has existed from the earlier trials which have focused purely on activity and fitness levels which are not directly correlated to fatigue levels or indeed an outcome that patient's value [Citation37].
An early example of a more pragmatic approach investigated the effectiveness of an oncologist exercise recommendation, compared to the usual care of referral to an exercise specialist [Citation38].This showed that the group receiving the oncologist recommendation reported significantly greater exercise participation than usual care at five-weeks. The advantages of using services and professionals where patients are already involved are much more likely to have greater adherence and to be able initiate an intervention at an earlier stage. Fatigue was also reduced in both groups but was not significantly different – the earlier involvement of an intervention that reduces fatigue is a significant advantage. This is important as there some evidence that reducing peak fatigue on treatment may extrapolate to a reduce incidence of long-term fatigue [Citation6,Citation23].
A review of psychosocial interventions to reduce fatigue during treatment [Citation39] have demonstrated the effectiveness of approaches to promote physical activity using techniques, such as motivational interviewing; behavioural counselling based on stages of change; and counselling and behavioural modification. These types of trial design have an overlap with other complex interventions, such as cognitive behavioural therapy, and may be augmented by telephone or online counselling. Many of these types of trials have attempted to use a group-based approach rather than individual, however, the level of uptake has been consistently poor. Additionally the use of multiple outcomes has meant that improvement in fatigue has often been a secondary effect. All of these trials demonstrated positive increases in physical activity measured either in terms of regular self-assessed exercise participation, or minutes of activity measured by accelerometer. The relationship between these self-reported or objective changes in activity and fatigue is still complex. There may be biological and/or psychological mediators from exercise but the specific elements have yet to be clearly delineated [Citation32].
Many of the trials of exercise for fatigue included in recent systematic reviews [Citation11,Citation24,Citation27] demonstrated the effectiveness of a supervised exercise groups. There was no overall difference in fatigue improvement between home- or gym-based trials and no significant differences between low and high intensity training. In addition there is a significant question remaining over longer term adherence and what level/frequency of exercise is required to reduce fatigue. While supervised exercise and use of gyms is one avenue, this approach will not suit all as we will now discuss.
Adherence to exercise and longer term follow-up to minimise chronic fatigue
One potential method to be explored is the UK National Health Service (NHS) Physical activity care pathway ‘Let's Get Moving’ (). The Let's Get Moving model enables healthcare professionals, as part of a standard consultation, identify people who would benefit from becoming more active, then using a shared decision making guiding style raise the importance of moving more, share the benefits, and for those interested then either signpost onto further support or those not revisit at a later date. This could be very brief advice, a 30 second intervention or brief advice about a 5 minute intervention. For those interested in more support, a suitable professional (e.g. nurse, allied health care or exercise professional) can then provide tailored support with ongoing follow-up to enable long-term behaviour change. This brief intervention includes techniques to build self-efficacy including goal setting and signposting to local affordable specialist and non-specialist physical activity opportunities that are tailored to the individual's needs. This could include walking groups (e.g. walking for health, paths for all – both UK based although similar organisations will exist in other countries), exercise on referral, graded exercise therapy. The NICE guidance on behaviour change states that there should be regular follow-up for 12 months [Citation30]. The relationship to fatigue is clear – there is consistent evidence that exercise reduces fatigue and that mediation of fatigue can occur with low intensity interventions. A method that can be incorporated into routine clinical practice has the potential to have a significant impact.
Figure 1. An illustration to demonstrate the cyclical (non-linear) nature of this service approach. This image is taken from the NHS Physical Activity Care Pathway “Let’s Get Moving” commissioning guidance
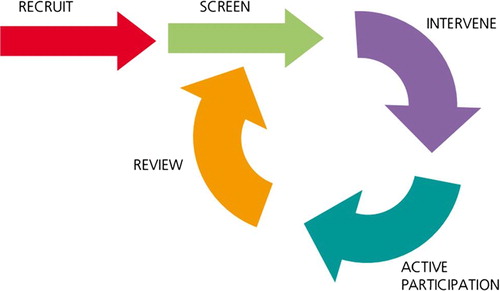
Pedometers could be provided to assist with self-monitoring of activity levels. Self-report logs of daily activity/walking, pedometer steps, and ratings of perceived exertion can be completed and related to fatigue levels over a period of months with regular contact to promote behavioural change and ongoing feedback. This model has been suggested by others in designing future trials and on the current evidence ought to be more widely adopted [Citation31].
This model of care is being tested as part of an integrated solution to cancer care in a number of clinical settings across the UK at present. These are being evaluated using adapted version, specifically tailored to cancer, from the Public Health England standard evaluation framework for physical activity [Citation30]. This model is not unique to the UK and is used for illustrative purposes – similar schemes will be in place across all developed countries.
Outcome measures in addition to fatigue
It is important to have an agreed outcome measure for these types of interventions – not be simply limited to fatigue but a broader quality of life measure and occupational and economic impact [Citation40]. It ought to be possible to link this to an objective measure of activity through the growing number of activity monitors although this may still be limited to a research setting. The data will have a role for individual goal setting and feedback which in turn may propagate behavioural change and minimise fatigue.
The use of motivational interviewing behavioural counselling and tailored interventions based on stages of change/trans theoretical model seemed to offer most benefit for longer term change.
Most of the effective interventions were based on behavioural theory, such as social-cognitive theory or the trans-theoretical model, and offered physical activity that was closely tailored to the patient's needs and capabilities. This approach has been successfully used in chronic fatigue syndrome [Citation34] and elements could readily be applied to cancer patients – potentially at all stages of treatment and after completion.
Future research
The use of self-reported objective exercise ought to be increasingly straightforward with the increased use of technology and using of exercise monitoring on a phone application. Harnessing this data even in the absence of any intervention would provide an objective study of activity during treatment that could be correlated with intermittent patient reported and recorded measures of fatigue. This will be especially important for patients undergoing complex or prolonged treatment regimens (most notably haematological cancers). We are still not readily monitoring fatigue or its relationship with activity during treatment. The link to translational research is still missing. We need to be routinely collecting samples in order to monitor the exercise ‘dose response’ and to identify mediators and moderators of fatigue. These measures should also be included in clinical trials as a far as possible within a core set of patient reported outcome domains.
There is a clear need for further research into a scalable intervention, such as the NHS physical activity care pathway ‘Let's Get Moving’ that can be integrated into cancer care, at all stages of the cancer care pathway across secondary and primary care. We also need to specifically monitor the effects of this on fatigue levels both acutely and as a later effect. There is, however, still a need to evidence both its effectiveness in enabling people to change their behaviour, the cost effectiveness of intervention, and the feasibly for maintained fatigue reduction.
Conclusions
We have discussed in our review potential tools and resources to support practitioners deliver exercise interventions in a high quality and systematic way, made as simply as possible in order to have the maximal impact on fatigue reduction acutely on treatment.
The potential increasing costs of chronic fatigue in an increasing number of patients mean it is now essential to integrate the evidence of physical activity, and effective interventions into clinical practice in a systematic way. This is illustrated through the updated NICE clinical guidance for prostate cancer [Citation41] that includes a recommendation to: ‘Offer men who are starting or having androgen deprivation therapy supervised resistance and aerobic exercise at least twice a week for 12 weeks to reduce fatigue and improve quality of life’. This recommendation is made in the context of an entire overview of the diagnosis and management of prostate cancer. This appears to highlight the importance of fatigue from both a professional and patient perspective. However, within the guidance there is no support in how to do this in a high quality way and this is an ongoing limitation to work in this area. Fatigue while being a ubiquitous problem does not have the same specific treatments as other symptoms.
In summary we would like to raise awareness of the simple steps anyone can take to make the most of the interventions previously outlined to improve fatigue and other related symptoms for an overall improved quality of life. Once we build on more simple interventions for all levels of fatigue, we can use internationally agreed protocols to design trials and outcomes for those with more severe fatigue and further elucidate the exact mechanisms. This will lead to more targeted interventions with increased effect sizes and impact.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Minton O, Berger A, Barsevick A, Cramp F, Goedendorp M, Mitchell SA, et al. Cancer-related fatigue and its impact on functioning. Cancer 2013;119:2124–30.
- Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
- Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 2010;116: 5740–8.
- Jacobsen PB, Donovan KA, Small BJ, Jim HS, Munster PN, Andrykowski MA. Fatigue after treatment for early stage breast cancer: A controlled comparison. Cancer 2007; 110:1851–9.
- Hansen JA, Feuerstein M, Calvio LC, Olsen CH. Breast cancer survivors at work. J Occup Environ Med 2008;50: 777–84.
- Bower JE. Cancer-related fatigue mechanisms, risk factors, and treatments. Nature Rev Clin Oncol 2014;11:597–609.
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
- Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs 2010;33:201–12.
- Wielgus KK, Berger AM, Hertzog M. Predictors of fatigue 30 days after completing anthracycline plus taxane adjuvant chemotherapy for breast cancer. Oncol Nurs Forum 2009;36:38–48.
- Fan HGM, Park A, Xu W, Yi QL, Braganza S, Chang J, et al. The influence of erythropoietin on cognitive function in women following chemotherapy for breast cancer. Psychooncology 2009;18:156–61.
- Tomlinson D, Diorio C, Beyene J, Sung L. Effect of exercise on cancer-related fatigue: A meta-analysis. Am J Phys Med Rehabil 2014;93:675–86.
- Loy BD, O’Connor PJ, Dishman RK. The effect of a single bout of exercise on energy and fatigue states: A systematic review and meta-analysis. Fatigue Biomed Health Behav 2013;1:223–42.
- Storey DJ, Waters RA, Hibberd CJ, Rush RW, Cargill AT, Wall LR, et al. Clinically relevant fatigue in cancer outpatients: The Edinburgh Cancer Centre symptom study. Ann Oncol 2007;18:1861–9.
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008;26: 971–82.
- Alexander S, Stone P, White S, Andrews P, Nussey S, Bano G. Evaluation of central serotonin sensitivity in breast cancer survivors with cancer-related fatigue syndrome. J Pain Symptom Manage 2010;40:892–8.
- Berger A, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer 2010;18:105–14.
- Lerner M, Wigal T. Long-term safety of stimulant medications: Used to treat children with ADHD. J Psychosoc Nurs Ment Health Serv 2008;46:38–48.
- DuPont RL, Coleman JJ, Bucher RH, Wilford BB. Characteristics and motives of college students who engage in nonmedical use of methylphenidate. Am J Addict 2008;17:167–71.
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–31.
- Robb C, Haley WE, Balducci L, Extermann M, Perkins EA, Small BJ, et al. Impact of breast cancer survivorship on quality of life in older women. Crit Rev Oncol-Hematol 2007;62:84–91.
- Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv 2010;4:405–14.
- NCCN. Clinical practice guidelines in oncology. 2014 [cited 2014 Apr 10]. Available from: http://www.nccn.org
- Macmillan. The cancer and physical activity standard evaluation framework (CaPASEF) 2013 Available from: http://www.macmillan.org.uk/Documents/AboutUs/Health_professionals/Physicalactivity/Cancer-Physical-Activity-Standard-Evaluation-Framework-Measurement-Tools.pdf. Cited on 12th June 2014.
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;14 (11:CD006145:doi: 10.1002/14651858.CD006145.pub3.).
- Kampshoff C, Jansen F, van Mechelen W, May A, Brug J, Chinapaw M, et al. Determinants of exercise adherence and maintenance among cancer survivors: A systematic review. Int J Behav Nutr Phys Act 2014;11:80.
- Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J Natl Cancer Inst 2012;104:815–40.
- McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: A meta-analysis. Appl Physiol Nutr Metab 2011;36:892–903.
- Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J Natl Cancer Inst 2013;105:1821–32.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 2009;20:17–25.
- NICE. National insitute for health and clinical exc excellence – Behavioural change indiviudal approaches. Available from: https://www.nice.org.uk/guidance/ph49 2014.
- Chinapaw MM, Buffart L, van Mechelen W, Schep G, Aaronson N, van Harten W, et al. Alpe d’HuZes Cancer Rehabilitation (A-CaRe) research: Four randomized controlled exercise trials and economic evaluations in cancer patients and survivors. Int J Behav Med 2012;19:143–56.
- Buffart L, Galvao D, Brug J, Chinapaw M, Newton R. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev 2014;2:327–40.
- Howell D, Keller-Olaman S, Oliver T, Hack T. A pan- Canadian practice guideline and algorithm: Screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.
- White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011;377:823–36.
- Servaes P, Prins J, Verhagen S, Bleijenberg G. Fatigue after breast cancer and in chronic fatigue syndrome: Similarities and differences. J Psychosom Res 2002;52:453–9.
- Husebo A, Dyrstad S, Soreide J, Bru E. Predicting exercise adherence in cancer patients and survivors: A systematic review and meta-analysis of motivational and behavioural factors. J Clin Nurs 2013;22:4–21.
- Courneya K, Stevinson C, McNeely M, Sellar C, Friedenreich C, Peddle-McIntyre C, et al. Predictors of follow-up exercise behavior 6months after a randomized trial of supervised exercise training in lymphoma patients. Psychooncology 2012;21:1124–31.
- Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs 2005;9: 56–63.
- Goedendorp M, Gielissen M, Verhagen C, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009;(1): CD006953.
- Munir F, Yarker J, McDermott H. Employment and the common cancers: Correlates of work ability during or following cancer treatment. Occup Med 2009;59:381–9.
- NICE. Prostate cancer: Diagnosis and treatment. Available from: http://www.nice.org.uk/guidance/cg175 2014. Cited on 12th June 2014.
