Abstract
Background. Studies show a cross-sectional association between physical activity (PA) and fatigue among lymphoma cancer patients. However, few longitudinal studies have examined whether PA has a sustained effect on fatigue over time.
Purpose. To examine the longitudinal relationship between PA and fatigue.
Methods. All living individuals diagnosed with lymphoma between 1999 and 2010 as registered by the Dutch population-based Eindhoven Cancer Registry received a questionnaire on three time points. Generalized linear mixed models were used to estimate the independent effects of PA on fatigue.
Results. PA and fatigue levels did not differ between patients short-term (< 1 year) and long-term after diagnosis (1–5 years or > 5 years). PA behavior was relatively constant over time with 84% of the patients meeting the Dutch PA guidelines at all assessment periods. Fatigue scores also remained fairly stable over time with 46% of the patients never being fatigued and 29% always being fatigued. Multivariate analyses showed that patients who met the PA guidelines scored 6.2 (95% CI 3.1–9.2) points lower on total fatigue over time (between subject effect; p < 0.01), compared to patients not meeting PA guidelines.
Conclusion. During a period of two years, PA and fatigue levels were rather stable within lymphoma patients. Patients who met the PA guidelines reported lower levels of fatigue compared to those not meeting the guidelines, which appeared to be a constant association over time. The observed association between PA and fatigue underlines the importance to focus upon physical training in the care of cancer patients.
Due to the development of new therapies, survival rates of patients with lymphomas improved noticeably. In the Netherlands, five-year relative survival for patients diagnosed between 1989 and 1994 equaled 76% for Hodgkin lymphoma (HL), 66% for indolent non-Hodgkin lymphoma (NHL) and 41% for aggressive NHL. These rates increased to 81%, 76% and 48%, respectively, for patients diagnosed between 2001 and 2005. In 2012, there were approximately 5700 patients living with a history of HL and 28 400 with NHL [Citation1].
As the survival rate of these patients is increasing, attention should not only be given at curing the disease but also at the long-term consequences of the disease and its treatment. Fatigue is one of the most frequently reported and distressing symptoms among (long-term) HL and NHL patients [Citation2,Citation3]. Fatigue can be described as the perception of unusual tiredness that varies in pattern and severity and has a negative impact on the ability to function in people who have or have had cancer [Citation4]. Recent studies have reported percentages of fatigued HL patients up to 76% and 61% of NHL patients [Citation2,Citation3]. Prevalence rates of fatigue vary widely and depend on tumor type, sex, age and treatment modality, although the exact etiology of fatigue remains unknown. Fatigue has been reported to have a significant negative impact on perceived quality of life, even more than symptoms like nausea and pain [Citation5].
Several therapeutic strategies, pharmacological and non-pharmacological, have been proposed for the treatment of fatigue. A cross-sectional study showed that lower levels of physical activity (PA) were associated with more symptoms of fatigue among lymphoma patients [Citation6]. A recent systematic review showed that aerobic exercise training interventions seem to be feasible and safe and have positive effects on fatigue levels of lymphoma patients [Citation7]. Longitudinal studies among patients are needed to evaluate whether PA has a sustained effect on fatigue. Therefore, the aim of the current study is to examine the relationship between PA and fatigue over time.
Methods
Setting and population
This study is part of a dynamic, longitudinal, population-based survey among lymphoma patients registered within the Eindhoven Cancer Registry (ECR) of the Comprehensive Cancer Centre Netherlands. The ECR records data on all patients who are newly diagnosed with cancer in the southern part of the Netherlands, an area with 2.3 million inhabitants, 18 hospital locations and two large radiotherapy institutes. The ECR was used to select all patients who were diagnosed with indolent (including chronic lymphocytic leukemia) and aggressive B-cell non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL), as defined by the International Classification of Diseases for Oncology-3 codes (ICD-O-3), between 1 January 1999 and 1 July 2010. Ethical approval for the study was obtained from the local certified Medical Ethics Committee of the Maxima Medical Centre Veldhoven. Before the first assessment, the (former) treating physicians were asked to verify the selected patients’ study eligibility and in case patients were moved to another part of the Netherlands or received follow-up care at another institution, patients were classified as “patient with an unverifiable addresses”.
Data collection
Data collection was done within PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship). PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from the ECR. Details of the data collection method were previously described [Citation8].
In November 2009, patients diagnosed between January 1999 and January 2009 were included in the study and received the first questionnaire (time point 1; T1). In May 2010 and May 2011 patients newly diagnosed up to 1 July 2010 were subsequently invited to participate (time point 1; T1). Patients received subsequent questionnaires one year after the first one (time point 2; T2) and two years after the first (time point 3; T3).
Study measures
Socio-demographic and clinical characteristics. Clinical information was available from the ECR that routinely collects data on tumor characteristics, including date of diagnosis, tumor grade, histology, stage, primary treatment, and patients’ background characteristics. Comorbidity at the time of survey was categorized according to the adapted Self- administered Comorbidity Questionnaire (SCQ) [Citation9]. Questions on sociodemographics were added to the questionnaire.
Fatigue. Fatigue was assessed with the Fatigue Assessment Scale (FAS), a questionnaire consisting of 10 items. The response scale is a five-point scale (1 never to 5 always) and total scores can range from 10 to 50. Participants can be divided into two groups based on total FAS scores [Citation10]: not fatigued (as defined by a score of 10–21) and fatigued (22–50). The psychometric properties are good, with a Cronbach's alpha of 0.87 in the Dutch population, and pattern of correlations and factor analysis showing good convergent and divergent validity [Citation11].
Physical activity. PA was assessed with questions derived from the validated European Prospective Investigation into Cancer (EPIC) Physical Activity Questionnaire [Citation12]. Participants were asked how much time they spend on the following activities (average number of hours per week, in summer and winter separately): walking, bicycling, gardening, housekeeping, and sports. To include an estimate of intensity, metabolic equivalent intensity values (MET value: 1 MET = 4.184 kJ/kg body weight/h) were assigned to each activity, according to the compendium of physical activities [Citation13]. Total PA was calculated by summing h/wk of all activities. The duration of moderate to vigorous physical activity (MVPA) was assessed as time (h/wk) spent on walking, bicycling, gardening and sports (≥ 3 MET), excluding housekeeping and light intensity sports (< 3 MET). MVPA was dichotomized into meeting the Dutch PA guideline of 150 minutes per week or not [Citation14].
Statistical analyses
Differences in sociodemographic and clinical characteristics between respondents and non-respondents or patients with unverifiable addresses and between patients who completed one or more questionnaires were compared with a χ2 or ANOVA, where appropriate.
All other analyses were based on patients who completed at least two questionnaires. Differences between patients meeting and not meeting the PA guidelines at each time point were determined by t-tests. Differences between patients short- and long-term after diagnosis (< 1 year; 1–5 years; > 5 years) in levels of PA and fatigue levels were determined by ANOVA. Differences between patients according to stability of their PA behavior (never meeting, always meeting PA guidelines, fluctuating) in fatigue levels were also determined by ANOVA.
Generalized linear mixed models with random intercepts were used to estimate the independent inter-individual (between-patients changes) and intra-individual (within-patient changes) effects of PA on fatigue. Models were adjusted for factors determined by a priori hypotheses: sociodemographic (age, sex, educational level, marital status) and clinical (time since diagnosis, disease stage, chemotherapy, number of comorbid conditions) variables. Time was analyzed as regular categorical predictor with three levels (i.e. three time points). Sociodemographic and clinical characteristics were analyzed as time-invariant predictors (i.e. baseline characteristics were used). The inter-individual effect was represented by the difference between a patients’ average amount of PA reported during the study and the average level of PA of the total group. The intra-individual effect was represented by the difference between a patients’ PA level at one time point and his/her average PA level during the study. The association of PA and fatigue was assessed including PA as continuous variable (h/wk) (model I) and dichotomously (meeting guidelines yes/no; model II). In order to correctly interpret all model parameters, all continuous variables have been grand-mean centered.
All statistical tests were two-sided and considered significant if p < 0.05. All analyses were conducted using SPSS version 19.0 (Statistical Package for Social Sciences, Chicago, IL, USA). Clinically meaningful differences in FAS scores were determined with Norman's “rule of thumb”, whereby a difference of ≈0.5 standard deviation indicates a threshold of discriminant change in scores of a chronic illness [Citation15].
Results
Sociodemographic and clinical characteristics of respondents and non-respondents
At T1 the response rate was 78% (851 of the 1095 totally invited patients responded). Patients with unverifiable addresses (n = 315) received chemotherapy less often (55% vs. 61% and 67%) compared to non-respondents (n = 244) and respondents (p < 0.01). Non-respondents were younger (56 vs. 61 and 62 years) and diagnosed longer ago (4.5 vs. 3.9 and 3.7 years) compared to respondents and patients with unverifiable addresses (both p < 0.01). No differences between the three groups were seen with respect to disease stage, sex and radiotherapy. Fifty-five percent (n = 471) completed the questionnaire at T2 and 36% (n = 308) at T3.
Differences between lymphoma patients who completed one or more than one questionnaire
Of the 851 responders, 368 completed only one questionnaire (43%), 178 completed two questionnaires (21%) and 305 completed all three questionnaires (36%). No differences were seen between respondents who completed only the first (“baseline”) questionnaire and those who subsequently completed two or more questionnaires with respect to sociodemographic and clinical characteristics (). Patients who completed only one questionnaire less often reported to be drinking alcohol compared to those who completed two or more questionnaires (66% vs. 70%; p = 0.03). No significant differences between the two groups were observed regarding BMI, smoking status and PA levels. Patients who completed only one questionnaire were more often classified as fatigued (47% vs. 39%; p = 0.04) as compared to those who completed two or more questionnaires.
Table I. Sociodemographic and clinical characteristics of patients who completed only one or two or more questionnaires+.
Physical activity. Although patients shortly after diagnosis (< 1 year; 9.1; 8.3; 9.3 h/wk) reported less hours of PA per week compared to patients 1–5 years (11.6; 12.9; 12.9 h/wk) and > 5 years since diagnosis (10.0; 11.9; 11.4 h/wk) at all three time points, these differences were not statistically significant. Levels of PA were relatively stable as most patients (84%) were meeting the Dutch PA guidelines of 150 minutes of MVPA at all three time points, 3% never met the guidelines and 12% had fluctuating PA levels. Patients who never met the guidelines and those with fluctuating PA levels were older (70.9, 65.8 vs. 59.3 years; p < 0.01) and reported more often two or more comorbid conditions (64%, 57% vs. 33%; p = 0.01) compared to patients who always met the PA guidelines. Patients who never met the guidelines more often had a lower educational level compared to patients with fluctuating and patients who always met the guidelines (33% vs. 16% and 9%). No differences were seen between the groups with respect to time since diagnosis, sex, treatment and disease stage. Mean PA levels were highest for the patients always meeting the guidelines (12.5 h/wk), followed by patients with fluctuating PA behavior (4.3 h/wk) and patients who were never meeting the guidelines (0.6 h/wk; p < 0.01).
Fatigue. Although patients shortly after diagnosis (< 1 year; 19.5; 19.9; 20.8) reported lower mean levels of fatigue compared to patients 1–5 years (21.3; 21.9; 23.8) and > 5 years after diagnosis (21.1; 22.7; 22.9) at all three time points, these differences were not statistically significant. Levels of fatigue were relatively stable over time with 46% of the patients never being fatigued, 29% always being fatigued, 25% had fluctuating fatigue levels. No differences were found between the fatigue groups (never fatigued, always fatigued, fluctuating fatigue levels) with respect to age, time since diagnosis, sex, treatment and disease stage. Patients who were always fatigued more often reported two or more comorbid conditions compared to patients who were never fatigued or with fluctuating fatigue levels (55% vs. 27% and 37%; p < 0.001); and patients who were never fatigued less often had a lower educational level compared to patients who were always fatigues and with fluctuating fatigue levels (6% vs. 15% and 18%; p < 0.001).
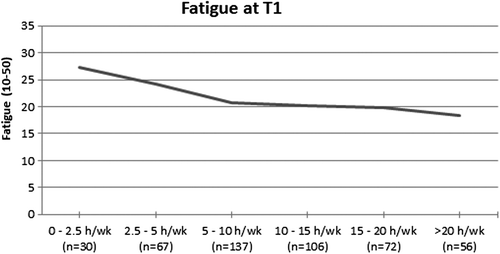
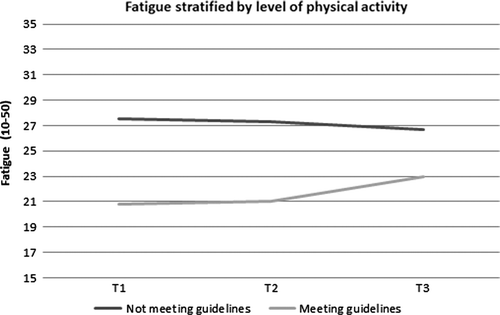
Physical activity and fatigue. At T1, patients reporting the highest levels of PA showed lowest levels of fatigue (; p < 0.001). Significant differences, being of clinical importance, were seen between patients who did and did not met the PA guidelines at T1 and T2 (; all p < 0.001). At T3, fatigue slightly increased in the active group, making the differences between the active and inactive group not statistically significant nor clinically relevant anymore.
Figure 2. Levels of fatigue stratified by level of physical activity. At T1 and T2 the difference between the groups were statistically significant (p < 0.001), at T3 the difference was not significant anymore.
Significant differences were found between the three stability groups (always meeting; never meeting guidelines and fluctuating PA behavior) at T1 and T2 on all fatigue scales (data not shown; all p < 0.05), whereby patients always meeting the guidelines had the lowest levels of fatigue and patients never meeting the guidelines had the highest levels of fatigue. These differences were of clinical importance for never meeting versus always meeting the PA guidelines.
Independent inter- and intra-individual association between physical activity and fatigue
With each additional hour of PA per week, the total fatigue level decreased approximately 0.2 points (95% CI 0.1–0.3; p > 0.001), after controlling for sociodemographic and clinical characteristics and time of assessment (model I).
Model II showed that patients who met the PA guidelines scored, on average, 6.2 (95% CI 3.1–9.2) points lower on total fatigue (p < 0.01). At T3 mean levels of fatigue were 2.6 points higher compared to T1 and 1.7 points higher compared to T2 (p < 0.05). Higher levels of fatigue were associated with lower educational level (β = 5.2 compared to high educational level), comorbid conditions (β = 1.6) and lower age (β = −0.2; all p < 0.01).
In both models, no intra-individual effects were found. No significant moderating effects of time on the association between PA and fatigue were found, indicating that the associations are likely to be stable over time.
Discussion
The aim of this longitudinal population-based study was to examine the relationship between PA and fatigue over time among lymphoma patients. PA and fatigue levels did not differ between patients short-term (< 1 year) and long-term after diagnosis (1–2 years or > 5 years). PA behavior was relatively constant over time with 84% of the patients meeting the Dutch PA guidelines at all assessment periods. Fatigue scores also remained fairly stable over time with 46% of the patients never being fatigued and 29% always being fatigued. Patients who met the PA guidelines had clinically relevant lower total fatigue scores. In multivariate analyses a significant between-subject effect was found for levels of moderate to vigorous PA and fatigue, indicating that patients who were more active over time reported lower levels of fatigue.
Our results are in line with those of other studies among different groups of cancer patients, as reported in two meta-analyses, showing that PA has a moderate beneficial effect on fatigue (effect sizes −0.30 – −0.38) [Citation16,Citation17]. However, research among lymphoma patients is scarce. A previous study among long-term HL survivors (> 5 years since diagnosis) reported a significant association between increased fatigue and lower (not further defined) exercise frequency [Citation6]. Another study among 438 NHL survivors found significantly lower levels of fatigue for survivors post-treatment who met the public exercise guidelines compared to those not meeting the guidelines, this relationship was not found among patients during treatment [Citation18]. Another cross- sectional study found no significant differences in the level of PA between 143 HL patients with chronic fatigue and 333 HL patients without chronic fatigue [Citation19]. Furthermore, a randomized controlled trial examined the effect of a 12-week aerobic exercise training on fatigue. There was a significant group difference for levels of fatigue, which were better for the intervention group compared to the control group [Citation20]. In an uncontrolled trial, nine HL patients with chronic fatigue who received a 20-week home-based exercise program showed significantly improved fatigue levels [Citation21].
We found a significant between-patient effect of PA on fatigue, but within-patient effects were not found. A possible explanation for the absence of this effect is the stability over time of both PA and fatigue. A recent review study indicated that 21–29% of the lymphoma patients met the American College of Sports Medicine PA guidelines [Citation7], this was approximately 87–90% in our study. Although most of the participants in our study were sufficiently active, there still remains room for improvement for these patients, as our analyses also showed that fatigue improved with each additional hour of PA. It could also be that exercise (specifically planned, structured, and repetitive training) has larger beneficial effects on fatigue than PA (activities that get the body moving such as gardening) alone. More research is needed to elucidate the optimal dose, type, timing and length of exercise to prevent and/or mitigate chronic fatigue among cancer patients [Citation22].
The observed association between PA and fatigue underlines the importance to focus upon physical training in the care of cancer patients. Exercise programs prove to be very effective in increasing muscle strength and physical fitness [Citation23]. However embedding of exercise programs in current oncologic rehabilitation guidelines is still not standard. Additionally, patients who are inactive are probably not likely to become spontaneously active. Pathophysiological or mental barriers or fatigue by itself could hinder patients from becoming more active [Citation24]. Adequate information provision by healthcare professionals about the benefits of PA to reduce fatigue seems necessary. Besides this, most exercise interventions are focused at short-term outcomes, while most patients will relapse into their “old” less active behavior in the long run. Therefore future PA interventions should include successful behavioral components into their program to increase the likelihood of structured PA behavioral changes. Additionally, a clearer understanding of the biology and genetics of fatigue will help the development of future personalized interventions, whereby physical training may be a helpful intervention for most patients given the trainability of several of the underlying factors, while in some patients with a certain distinct profile (also) pharmacological or psychological interventions will be indicated.
This is the first population-based study among lymphoma patients reporting on the prospective association between PA and fatigue. Some limitations of the present study should be mentioned. First, although information was present regarding demographic and clinical characteristics of the respondents and non-respondents, it remains unknown why non-respondents declined to participate. It could be that fatigued patients were more often non-respondents in our study, as we also showed that patients who completed only one questionnaire were more fatigued than those who completed two or more questionnaires. Fatigue could therefore be a reason for attrition. When the more fatigued patients who completed only one questionnaire would have participated in the other two assessments, we are expecting to find an even stronger association between PA and fatigue. Second, although our study has a longitudinal study design it is still not possible to determine causality among the study variables. Fatigue could be a barrier to become physically active, while the other way around regular exercise could also result in reduced fatigue levels. Furthermore, both PA levels and fatigue, could be influenced by variables that were not measured in this study. Another limitation is the use of a self-reported questionnaire to assess PA, which is susceptible to recall and social desirability. This may have led to an overestimation of PA and therefore absolute PA levels should be interpreted with caution. Future studies with objective physical activity measures (e.g. accelerometers in combination with a heart rate monitor) need to be conducted to test our findings. Nevertheless, our results are in accordance with another Dutch study showing that two years after diagnosis, Dutch breast cancer survivors reported spending 24.2 h/wk on total PA, and 9.8 h/wk on MVPA [Citation25]. In addition, the levels of MPVA of lymphoma survivors in our study seem comparable to a reference sample of Dutch people aged 65 years and older, who reported spending on average 90 min/day on MVPA (www.cbs.nl). However, they used a different questionnaire which hampers direct comparison of MVPA levels. In addition, our study sample is relatively healthy with respect to PA; which might indicate survivorship bias, since unhealthy lifestyles are related to mortality among lymphoma patients. This means that the survivors who completed more than one questionnaire in this study are more physically active because the inactive survivors only filled out one questionnaire or died earlier after their diagnosis. Another explanation for the high PA levels may be the active lifestyle of Dutch people after retirement, 66% of the study population was retired at the first assessment time. In addition, being diagnosed with lymphoma probably made people more aware of the importance of enough physical activity. Last, because all participants were lymphoma survivors, we can only generalize our results to this group of survivors.
To conclude, this study showed that the positive association between PA and lower levels of fatigue among lymphoma patients is consistent over time. Patients who are meeting the PA guidelines report lower levels of fatigue compared to those not meeting the guidelines. Within patients fatigue and PA levels are relatively stable over time.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cijfers over kanker. 2014. [cited 2014 Mar 27]. Available from: http://www.cijfersoverkanker.nl.
- Oerlemans S, Mols F, Issa DE, Pruijt JHFM, Peters WG, Lybeert M, et al. A high level of fatigue among long-term survivors of non-Hodgkin’s lymphoma: Results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica 2013;98:479–86.
- Daniels LA, Oerlemans S, Krol ADG, van de Poll-Franse LV, Creutzberg CL. Persisting fatigue in Hodgkin lymphoma survivors: A systematic review. Ann Hematol 2013;92: 1023–32.
- Barsevick AM, Cleeland CS, Manning DC, O’Mara AM, Reeve BB, Scott JA, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage 2010;39:1086–99.
- Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: Evolving concepts in evaluation and treatment. Cancer 2003;98:1786–801.
- Ng AK, Li S, Recklitis C, Neuberg D, Chakrabarti S, Silver B, et al. A comparison between long-term survivors of Hodgkin’s disease and their siblings on fatigue level and factors predicting for increased fatigue. Ann Oncol 2005; 16:1949–55.
- Vermaete N, Wolter P, Verhoef G, Gosselink R. Physical activity, physical fitness and the effect of exercise training interventions in lymphoma patients: A systematic review. Ann Hematol 2013;92:1007–21.
- van de Poll-Franse LV, Horevoorts N, Eenbergen MV, Denollet J ,Roukema JA, Aaronson NK, et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: Scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 2011;47:2188–94.
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63.
- Michielsen HJ, Drent M, Peros-Golubicic T, De Vries J. Fatigue is associated with quality of life in sarcoidosis patients. Chest 2006;130:989–94.
- Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res 2003;54:345–52.
- Pols MA, Peeters PH, Ocke MC, Slimani N, Bueno-de-Mesquita HB, Collette HJ. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int J Epidemiol 1997;26(Suppl 1):S181–9.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 Suppl):S498–504.
- NNGB, 2014. [cited 2014 Mar 27]. Available from: http://www.sportzorg.nl/bibliotheek/nederlandse-norm-gezond-bewegen-nngb/.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 2003; 41:582–92.
- Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: A meta-analysis. Am J Prevent Med 2012;43:e1–24.
- Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
- Vallance JK, Courneya KS, Jones LW, Reiman T. Differences in quality of life between non-Hodgkin’s lymphoma survivors meeting and not meeting public health exercise guidelines. Psychooncology 2005;14:979–91.
- Oldervoll LM, Loge JH, Kaasa S, Lydersen S, Hjermstad MJ, Thorsen L, et al. Physical activity in Hodgkin’s lymphoma survivors with and without chronic fatigue compared with the general population – a cross-sectional study. BMC Cancer 2007;7:210.
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol 2009;27:4605–12.
- Oldervoll LM, Kaasa S, Knobel H, Loge JH. Exercise reduces fatigue in chronic fatigued Hodgkins disease survivors – results from a pilot study. Eur J Cancer 2003; 39:57–63.
- Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol 2013;52:195–215.
- De Backer IC, Vreugdenhil G, Nijziel MR, Kester AD, van Breda E, Schep G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: Persistent improvement of physical performance and quality of life. Br J Cancer 2008;99:30–6.
- Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Vallance JKH, et al. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med 2005; 29:147–53.
- Voskuil DW, van Nes JG, Junggeburt JM, van de Velde CJ, van Leeuwen FE, de Haes JC. Maintenance of physical activity and body weight in relation to subsequent quality of life in postmenopausal breast cancer patients. Ann Oncol 2010; 21:2094–101.