Abstract
Background. The objective of this study was: 1) to compare health-related quality of life (HRQoL) scores of breast cancer survivors to matched controls; and 2) to examine the relative impact (explained variance) of the type and number of co-morbidities on HRQoL.
Material and methods. Data from the KARMA project was used in this cross-sectional study. For each woman diagnosed with breast cancer (n = 2552) there were two healthy age- and geographically matched females (n = 5104). Breast cancer survivors were categorized according to time since diagnosis: recently diagnosed (0–1 year), short- (2–5 years), mid- (6–10 years), and long-term survivors (> 10 years). Women completed a questionnaire addressing demographics (age, educational level, and geographical location), lifestyle factors (body mass index (BMI) and smoking), co-morbidities, and HRQoL. The difference in explained variance in six HRQoL-domains between demographics, lifestyle factors, and co-morbidity in women with breast cancer and matched controls was examined by hierarchical regression analyses.
Results and conclusion. Women recently diagnosed (n = 63), reported the worst HRQoL followed by short-term survivors (2–5 years, n = 863). Thereafter, HRQoL scores further improved (6–10 years, n = 726), and were comparable to healthy females after 10 years (n = 893). Co-morbidity has a negative impact on HRQoL, which increased with time after diagnosis. Cardiovascular disease and depression were the strongest associates. Breast cancer survivors report clinically significant improvement in HRQoL scores six years after diagnosis. Co-morbidity has a negative impact on HRQoL, which increases with time after diagnosis, even though the number of co-morbidities remains stable. In long-term survivors there should be increasing awareness of co-morbidity and its impact on HRQoL.
Over the past few decades, breast cancer treatments have become more effective. As a result the number of survivors has increased dramatically [Citation1]. Within the field of oncology, there has become increasing awareness for long-term outcomes, such as health-related quality of life (HRQoL) [Citation2]. Ample research has been conducted examining HRQoL among breast cancer patients and short- and long-term survivors [Citation3–7]. However, very few studies have examined the entire scope of patients ranging from recently diagnosed to survivors up to more than 10 years after diagnosis.
Worldwide the population is ageing, which leads to an increase in individuals with one or more chronic diseases [Citation8]. Together with increased survival rates, this brings a new challenge: a growing number of breast cancer survivors with one or more co-morbid conditions [Citation9]. In general, few studies have addressed the impact of co-morbidity on HRQoL in cancer patients or survivors [Citation10–12]. More specifically, studies targeting this issue in breast cancer are rare, yet very important given that the number of breast cancer survivors with co-morbid conditions is expected to grow rapidly over the next few decades.
The focus of this cross-sectional study in breast cancer survivors was therefore on HRQoL-scores for recently diagnosed and short-, mid-, and long-term survivors. Moreover, we examined the impact co-morbid conditions have on HRQoL. Our specific objectives were: 1) comparing HRQoL scores of age- and geographically matched controls to breast cancer survivors (i.e. 0–1, 2–5, 6–10, > 10 years after diagnosis); and 2) examining the relative impact (i.e. explained variance) of the type and number of co-morbidities on HRQoL on top of demographics and lifestyle factors.
Material and methods
Study population and procedure
Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA, www.karmastudy.org) comprised 70 561 women who attended mammography screening at one of four Swedish hospitals in the period between January 2011 and August 2013. The national mammography screening program invites all women at 18 month intervals for those 40–55 years, and for those between 56 and 74 years at 24 months. Each woman who attended mammography screening or a clinical mammography at one of the four hospitals received a letter inviting them to participate in KARMA one week after their call for mammography screening. At each visit blood is donated which is processed at the Karolinska biobank and participants were asked to complete a comprehensive online survey, entailing more than 250 questions. The online survey addressed breast cancer diagnosis (yes/no), year of (first) breast cancer diagnosis and health-related issues (e.g. lifestyle factors; previous medical conditions other than breast cancer; medication use; and HRQoL). Unfortunately, no information such as age or having ever received a (breast)cancer diagnosis is available for the non-responders. In the present study, we included all women who were ever diagnosed with breast cancer from the KARMA database. In addition, for each breast cancer survivor, two age- and geographically matched healthy controls – that is women who were never diagnosed with cancer – were selected from the KARMA database.
The KARMA study was approved by the Swedish regional ethical board at the Karolinska Institutet and is conducted in accordance with the Declaration of Helskini [Citation13].
Measurements
Demographics and lifestyle factors
Women's age was calculated by personal identification number, that is, date of birth and date of participation. All women reported their educational level and geographical location. Geographical location was operationalized as the three hospitals in the South of Sweden or the Stockholm hospital. Based on women's height in meters divided by their squared weight in kilogram, their body mass index (BMI) was calculated. The number of years smoking was self-reported.
Breast cancer and co-morbidities
By means of the online survey women reported whether they were ever diagnosed with breast cancer and if so when they were (first) diagnosed. Moreover, women reported on having one of the following co-morbid conditions; depression, diabetes, other cancers than breast cancer, myocardial infarction, angina, heart failure, and stroke. If women reported one or more of the latter four conditions, they were categorized as having cardiovascular diseases (CVD).
Health Related Quality of life (HRQoL)
HRQoL was measured with the European Organization for Research and Treatment of Cancer Quality of Life questionnaire Core 30 (EORTC QLQ-C30) [Citation14]. It includes overall quality of life (that is, global health status), five functional scales (physical; role; emotional; cognitive; and social), three symptom scales (fatigue; nausea or vomiting; and pain), and six single items (dyspnea; insomnia; appetite loss; constipation; diarrhea; and financial difficulties). Symptoms of cancer and treatment are expected to diminish after treatment has been terminated [Citation12,Citation15]. Moreover, there is substantial overlap between the symptoms assessed by the EORTC QLQ-C30 and co-morbidities. We therefore included overall quality of life and the five functional scales in this study among survivors and healthy controls. These scales are linearly transformed from 0 to 100. High scores on the quality of life scale indicate a high level of HRQoL, and high scores on the functional scales indicate a high level of functioning. The EORTC QLQ-C30 is considered to have good psychometric qualities and has been validated [Citation14].
Statistical analyses
All breast cancer survivors were categorized in four groups according to time since diagnosis: recently diagnosed (0–1 year), short-term survivors (2–5 years), mid-term survivors (6–10 years), and long-term survivors (> 10 years). Number of co-morbidities were categorized in three groups: zero (0), one (1), or more than one (> 1) co-morbidity. Differences in demographics, lifestyle factors, and co-morbidities between breast cancer survivors and the age- and geographically matched controls were analyzed using ANOVA, χ2 or t-tests were appropriate. Significant differences were treated as possible confounders in the subsequent analyses.
By means of linear regression analyses, changes in HRQoL scores over time were analyzed by comparing scores among breast cancer survivors with varying time since diagnosis to matched controls, while controlling for the matching variables (age and geographical location) and possible confounders (educational level, number of years smoking, and number of co-morbidities). Statistically significant differences in HRQoL are not always clinically significant. Therefore, a scaled effect size (ScES) was calculated by dividing the difference between HRQoL-scores for breast cancer survivors and controls by the scale range [Citation6]. For example, the HRQoL-scales range from 0 to 100, if a mean score improves from 20 to 25, the ScES is 5/100 × 100 = 5%. ScES can be interpreted as clinically relevant when ScEs ≥ 5 [Citation16].
Finally, the impact of co-morbidities on HRQoL was tested in various ways. First, unadjusted HRQoL-scores were calculated for individuals with 0, 1 and > 1 co-morbidity for both the total group of breast cancer survivors and the controls. Second, hierarchical multivariable regression analyses were performed to assess the amount of variance in HRQoL explained by: 1) the number of co-morbidities (0, 1, > 1); and 2) the type of co-morbidity (other cancers, depression, diabetes, heart infarction, angina, and heart failure) on top of demographics and lifestyle factors. In the hierarchical regression analyses variables were entered in blocks. First, demographics (age, geographical location, and educational level) were entered as block 1. Then, lifestyle factors (BMI and number of years smoking) were added to the model in block 2. Finally, in block 3, co-morbidity (number of co-morbidities or type of co-morbidity) was added. The change in R2 was reported as the amount of variance explained per block. Explained variance (R2) was used as indicator of unique contribution to explaining variation in HRQoL. Studying the variance in HRQoL that is explained by co-morbidity, provides us with an effect size that shows the relative importance of co-morbidity in relation to demographics and lifestyle factors. Third, the hierarchical multivariable regression analyses addressing the impact of the number of co-morbidities on HRQoL were repeated for the subcategories of survivors. The prevalence of the type of co-morbidities over the subcategories of breast cancer survivors was too small to conduct the additional analyses. Since the number of patients included in this study was substantial (n = 2552 breast cancer survivors and n = 5104 controls), a conservative significance level of 0.01 (two-sided) was employed. Analyses were performed in SPSS.
Results
Response rate, demographics, lifestyle factors, and co-morbidities
For 65 981 (94%) of the 70 561 women in KARMA survey data was available. For the present analyses all women ever diagnosed with breast cancer (n = 2727; 4.1%) and two age- and geographically matched controls (n = 5454) were selected.
Demographics and lifestyle factors for breast cancer survivors and controls are presented in . The mean age for breast cancer survivors and controls was 62 years. As expected, long-term survivors were older (p < 0.01). Breast cancer survivors reported having smoked for a longer time than the matched controls (p < 0.01).
Table I. Demographics, lifestyle factors, and co-morbidities for the study population in frequencies and percentages or mean and standard deviation.
Healthy women reported less often having (17%) one or more co-morbidities then breast cancer survivors (25%) (). This difference was due to other cancers (ca. 10%) in our breast cancer survivors compared to no cancer diagnoses in our healthy women as that being ever diagnosed with cancer rephrased women from being included as healthy controls. Note that, other cancers could either be diagnosed prior to or after the breast cancer diagnosis. In both controls and survivors depression was most prevalent. The prevalence levels of both the number and the specific co-morbidities were constant across subgroups of cancer survivors.
Health-related quality of life over time
shows the general trend comparing the different subgroups of cancer patients/survivors to all controls. Women recently diagnosed with breast cancer (n = 63), reported the lowest levels of HRQoL, followed by short-term breast cancer-survivors (2–5 years, n = 863). Role and emotional functioning were similar after 6–10 years (n = 726) to the mean scores reported by the controls. Scores on the quality of life, cognitive and social functioning scales were comparable to healthy females after 10 years (n = 893).
Figure 1. General trend for HRQoL-scores for the subgroups of breast cancer survivors versus all controls. CF = cognitive functioning; EF = emotional functioning; PF = physical functioning; QL = quality of life (global health); RF = role functioning; SF = social functioning.
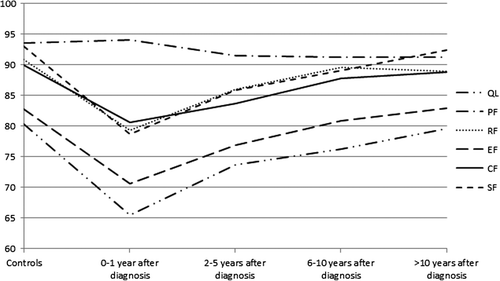
Furthermore, each subgroup of breast cancer survivors was compared to their matched control group, and adjusted scale means are presented in . Breast cancer survivors diagnosed within the last year reported clinically significant lower scores (difference > 10%) on five out of six HRQoL-scales. Short-term cancer survivors (2–5 years after diagnosis) also reported clinically different scores (ca. 5%), that is, lower HRQoL-scores. Although for four out of six HRQoL-scales, scores for mid-term breast cancer survivors (6–10 years after diagnosis) were statistically significantly lower than the HRQoL of their matched controls, these differences were not clinically significant. After 10 years HRQoL-scores for survivors were comparable to that of their matched controls with respect to all scales.
Table II. Comparison of HRQoL-scores for the subgroups of breast cancer survivors with their age- and geographically matched controls.
Impact of co-morbidity on health-related quality of life on top of demographics and lifestyle factors
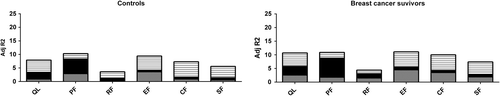
depicts the variance explained by demographics, lifestyle factors, and the number of co-morbidities for the healthy controls and the entire breast cancer sample. The total amount of explained variance in HRQoL was higher for breast cancer survivors (R2 range 4.4–11.1%) than for the controls (R2 range 3.6–10.3%). The reported number of co-morbidities had an equally high impact among controls (R2 range 2.0–5.7%) and among breast cancer survivors (R2 range 2.1–5.7%). Demographics had a greater impact on HRQoL in breast cancer survivors (R2 range 1.5–4.5%) than in controls (R2 range 0.1–3.6%). In detail, both healthy women and breast cancer survivors reported significantly lower HRQoL scores as the number of co-morbidities increase. Older age was related to a higher mental functioning (e.g. emotional, cognitive, and social functioning), but lower physical functioning. A higher BMI was significantly associated with lower levels of HRQoL. Women, who reported that they have smoked a longer period, scored lower on the HRQoL scales (data not shown).
Figure 2. Explained variance (R2 in percentages) of HRQoL-scores explained by demographics, lifestyle factors, and number of co-morbidities among controls and all breast cancer survivors. Dotted (bottom) block, demographics (age, educational level, and geographical location); black block, lifestyle factors (BMI and number of years smoking); stripped (upper) block, number of co-morbidities; CF = cognitive functioning; EF = emotional functioning; PF = physical functioning; QL = quality of life (global health); RF = role functioning; SF = social functioning.
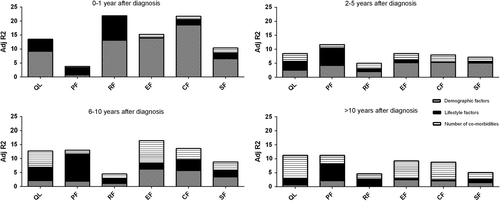
The analyses were repeated for the four different subcategories of cancer survivors (recently diagnosed and short-, mid-, and long-term survivors). The total amount of variance in HRQoL explained decreased over time, while the impact of the number of co-morbidities on HRQoL increased (). For recently diagnosed patients the impact of co- morbidities on HRQoL ranged from 0.0% to 1.8%. For short-term survivors the impact ranged from 1.2% to 2.8% and for mid-term survivors from 1.3% to 8.0%. For long-term survivors the impact of the number of co-morbidities on HRQoL further increased to 1.9–8.2%.
Figure 3. Explained variance (R2 in percentages) of HRQoL scores explained by demographics, lifestyle factors, and number of co-morbidities among the subgroups of breast cancer survivors (0–1 year, 2–5 years, 6–10 years, and > 10 years after diagnosis). Dotted (bottom) block, demographics (age, educational level, and geographical location); black (middle) block, lifestyle factors (BMI and number of years smoking); stripped (upper) block, number of co-morbidities. CF = cognitive functioning; EF = emotional functioning; PF = physical functioning; QL = quality of life (global health); RF = role functioning; SF = social functioning.
Impact of type of co-morbidities on health-related quality of life
When all co-morbidities were entered separately into the model, the total portion of explained variance in HRQoL increased with 1.6–2.8% for the control group and 0.5–2.5% for breast cancer survivors (). In both groups, most variance in HRQoL was explained by CVD or depression. The amount of variance (R2) explained by CVD's ranged from 0.3% to 1.8% for controls, and from 0.2% to 1.5% for breast cancer survivors. For depression scores ranged from 1.0% to 8.0% and 0.9–7.1% for controls and breast cancer survivors, respectively.
Table III. Explained variance (R2 in percentages) of HRQoL scores explained by demographics (age, educational level, and geographical location), lifestyle factors (BMI and number of years smoking), and type of co-morbidities among controls and all breast cancer survivors.
Discussion
This study showed that breast cancer survivors report a poorer HRQoL than healthy controls even 6–10 years after diagnosis, with clinically significant differences up to five years after diagnosis. Co- morbidity has a negative impact on HRQoL, which increased with time after diagnosis, even though the number of co-morbidities remained stable. The relative impact (i.e. explained variance) of the type and number of co-morbidities on HRQoL was, however, limited.
The presence of the different types of co-morbidities did not differ between controls and breast cancer survivors. This finding is unexpected as you would anticipate a higher prevalence of co-morbid conditions based on existing literature, since co-morbidities are often seen as adverse (late) effects of cancer-treatment and shared etiological factors are suggested for both cancer and co-morbid conditions [Citation17]. Negative effects on cardiovascular functioning are, for example, often reported as a result of cardiotoxic chemotherapy or radiation to the thorax [Citation18]. Moreover, obesity is seen as an etiological factor for both cancer and CVD [Citation17].
Breast cancer survivors reported lower HRQoL scores on all scales compared to healthy women. When looking at the various breast cancer survivor groups, HRQoL dropped during the first years after diagnosis. HRQoL scores improve thereafter, and for role and emotional functioning the scores were comparable to healthy females after 6–10 years. For quality of life, physical, cognitive, and social functioning scores, however, this process appeared to take an even longer period. Functioning was comparable to that of healthy females after 10 years or more. These results, that returning to HRQoL levels similar to that in the population takes several years, are supported by other studies. Studies comparing cancer patients’ short-term HRQoL have found lower levels of HRQoL than in the general population up to five years after diagnosis [Citation3–5]. In a review and recent longitudinal study of HRQoL in mid- and long-term breast cancer survivors more than five years after diagnosis, most included studies reported similar or higher levels of HRQoL as in the control groups [Citation6,Citation7].
Having one or more co-morbidities was related to significantly lower HRQoL scores for both breast cancer survivors and controls, which is in agreement with previous studies [Citation10,Citation15,Citation19]. Although the number of co-morbidities in our study sample did not increase with time since diagnosis, the effect on HRQoL did increase. This finding is in agreement with the results of a previous study of breast cancer survivors, 20 years from the onset of breast cancer treatment, where the impact of cancer and its treatment decreased over time and was largely replaced by the impact of age-related co-morbidities and functional decline [Citation10]
Demographic factors, with age in particular, had the greatest impact on HRQoL in recently diagnosed breast cancer patients. In other words, younger women reported lower HRQoL-scores then older women in the first years after diagnosis. Getting a breast cancer diagnosis and undergoing treatment may be particularly hard on young women, given their often challenging and demanding life (e.g. combining long work hours and child care). This is in agreement with a study by Ganz et al. who showed that the effect of cancer on educational and family plans, and the ability to take care of others including children is stronger in younger than in older women [Citation20].
The total amount of variance explained in this study was lower than in a comparable study among (non)Hodgkin's lymphoma, thyroid and colorectal cancer [Citation15], yet similar to a study in recently diagnosed breast cancer patients [Citation21]. There are many other factors, such as social support, for example, that could have impacted participants’ HRQoL [Citation22]. Moreover, several studies have shown that in addition to the disease or symptoms, the experienced symptom distress has a large negative impact on HRQoL [Citation10].
Co-morbidities were assessed by means of self-report. This may be an advantage as physician diagnosis and self-report shows a high level of agreement [Citation23], whereas co-morbidities are often underreported in registries [Citation24]. Our cross-sectional study design makes it unclear whether co-morbid conditions – including other cancers – were present before or after breast cancer diagnosis. Hence, the direction of the relation between HRQoL and co-morbidity remains unclear. Moreover, by virtue of remaining disease-free for more than 10 years, long-term survivors have a better prognosis, may have received less aggressive treatment, and are more likely to comply with screening, compared to women closer to their breast cancer diagnosis. Furthermore, only a limited number of recently diagnosed women were included, which may be due to toxicities related to active treatment. In addition, no information on demographics and (breast) cancer status of these non-attenders or non-participants was available, therefore the possibility of selection bias cannot be ruled out.
Finally, it is important to discuss the implications of the selection procedure for the interpretation and generalizability of our results. First, the mammography attendance rate was unknown, although on average 80% of women participate in the Swedish national breast cancer screening program [Citation25]. In our study that means that of the 207 662 women eligible for mammography screening in one of the four KARMA hospitals during the inclusion period, approximately 166 130 women (∼80%) attended screening. During the study period, 70 561 women – approximately 42% of the approximately 166 130 – were included in KARMA. Reasons for why women did not attend mammography screening or participated in the KARMA study are unknown. Second, we included two healthy age- and geographically matched controls which were not diagnosed with breast cancer nor received any other cancer diagnosis. Moreover, women attending one of four KARMA hospitals, either in Stockholm or South Sweden, were invited to participate. It is therefore unclear whether our results can be generalizable to women who do not have breast cancer but were ever diagnosed with other forms of cancer. Moreover, results may not be generalizable to women living in other parts of Sweden. Nonetheless, these effects of a possible selection bias and restricted generalizability are likely to be limited due to our case-control design.
This study has various strengths, such as the large number of breast cancer survivors and the inclusion of an age- and geographically matched control group. Although our study was cross-sectional, a wide range of breast cancer survivors were included, ranging from recently diagnosed to survivors up to more than 10 years after diagnosis. This cohort therefore encompasses various survivors who were treated with a wide range of treatments. Self-reported information on type of treatment was limited to 67% of the included survivors. Almost all of them (99%) were surgically treated. In addition, 35% and 73% received chemo- and/or radiotherapy. Adding information on type of treatment to the model relating co-morbidities to HRQoL did not increase the level of explained variance (data not shown). Type of treatment was moreover not statistically significantly related to HRQoL in the model.
In conclusion, the results of this study underscore the resilience of breast cancer survivors. Hence, HRQoL of breast cancer survivors is impaired during the first five years, but improve thereafter, and is comparable to healthy controls 6–10 years after diagnosis. Moreover, results display the importance of co-morbidity and its effect on HRQoL. Co-morbidity is an important research area, as it does not only impact HRQoL, but can also interfere with treatment and prognosis [Citation26]. Future research focussing on co-morbidities in breast cancer survivors is therefore warranted.
Acknowledgments
The present research is partly supported by a Social Psychology Fellowship from the Dutch Cancer Society to Dounya Schoormans (#UVT2013-5893) and a grant by FORTE awarded to Yvonne Brandberg and Kamila Czene [#2013-0474]. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, et al. EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Ann Oncol 2003; 14(Suppl 5):v128–49.
- Kaplan RM. Quality of life: An outcomes perspective. Arch Phys Med Rehabil 2002;83:S44–50.
- Glaser AW, Fraser LK, Corner J, Feltbower R, Morris EJ, Hartwell G, et al. Patient-reported outcomes of cancer survivors in England 1–5 years after diagnosis: A cross-sectional survey. Br Med J Open 2013;3:e002317.
- Lemasters T, Madhavan S, Sambamoorthi U, Kurian S. A population-based study comparing HRQoL among breast, prostate, and colorectal cancer survivors to propensity score matched controls, by cancer type, and gender. Psychooncology 2013;22:2270–82.
- Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It's not over when it's over: Long-term symptoms in cancer survivors – a systematic review. Int J Psychiatry Med 2010;40:163–81.
- Hsu T, Ennis M, Hood N, Graham M, Goodwin PJ. Quality of life in long-term breast cancer survivors. J Clin Oncol 2013;31:3540–8.
- Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: A systematic review. Eur J Cancer 2005;41:2613–9.
- WHO. Active ageing: a policy framework. Geneva: World Health Organization; 2002.
- Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit Rev Oncol Hematol 2005; 55:231–40.
- Cohen HJ, Lan L, Archer L, Kornblith AB. Impact of age, comorbidity and symptoms on physical function in long-term breast cancer survivors (CALGB 70803). J Geriatr Oncol 2012;3:82–9.
- Osthus AA, Aarstad AK, Olofsson J, Aarstad HJ. Comorbidity is an independent predictor of health-related quality of life in a longitudinal cohort of head and neck cancer patients. Eur Arch Otorhinolaryngol 2013;270:1721–8.
- Wahlgren T, Levitt S, Kowalski J, Nilsson S, Brandberg Y. Use of the Charlson Combined Comorbidity Index to predict postradiotherapy quality of life for prostate cancer patients. Int J Radiat Oncol Biol Phys 2011;81:997–1004.
- Rickham PP. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964;2:177.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Vissers PA, Thong MS, Pouwer F, Zanders MM, Coebergh JW, van de Poll-Franse LV. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: Analyses of data from the PROFILES registry. J Cancer Surviv 2013;7:602–13.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol 2007;46:417–32.
- Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol 2005;23:7685–96.
- Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: Quality of life of young breast cancer survivors. Psychooncology 2004;13:147–60.
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst 2002;94:39–49.
- Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 2006;107:2496–503.
- Kroenke CH, Kwan ML, Neugut AI, Ergas IJ, Wright JD, Caan BJ, et al. Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat 2013;139:515–27.
- Baumeister H, Kriston L, Bengel J, Harter M. High agreement of self-report and physician-diagnosed somatic conditions yields limited bias in examining mental-physical comorbidity. J Clin Epidemiol 2010;63:558–65.
- Powell H, Lim LL, Heller RF. Accuracy of administrative data to assess comorbidity in patients with heart disease: An Australian perspective. J Clin Epidemiol 2001;54: 687–93.
- Olsson S, Andersson I, Karlberg I, Bjurstam N, Frodis E, Hakansson S. Implementation of service screening with mammography in Sweden: From pilot study to nationwide programme. J Med Screen 2000;7:14–8.
- Lemmens VE, Janssen-Heijnen ML, Verheij CD, Houterman S, Repelaer van Driel OJ, Coebergh JW. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg 2005;92:615–23.
