Abstract
Background. Relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) after autologous stem cell transplantation (ASCT) remains a challenge. For these patients treatments with different mechanisms of action rather than classical chemotherapy are needed.
Patients and methods. Patients with R/R cHL after ASCT were recruited in a phase II trial (EUDRA CT: 2009-016588-12). Lenalidomide was administered at 20 mg/day for 21 days and cyclophosphamide at 50 mg/day for 28 days (cycles every 28 days). Dose escalation for lenalidomide was permitted. In 2009 we considered that this treatment would be promising if response rate were over 60% and a Simon two-stage binomial design was used to calculate the sample size. A total of 46 patients were planned but the trial would be stopped if less than seven responses after four cycles were obtained in the first 16 patients.
Results. The trial was closed early because only five responses were observed after four cycles in the first 16 patients included. Median age was 34 years (18–77). The median number of previous lines was five (2–6). At inclusion, 10 patients were primary refractory and 11 refractory to the last therapy. A total of 110 cycles were administered, with grade ≥ 3 toxicity in 43 cycles (39%). One non-neutropenic patient developed septic shock resulting in death. An ORR of 38% (1 CR and 5 PR) was observed and a total of 10 patients (62%) achieved clinical benefit. Median progression free survival and overall survival were seven and 19 months, respectively. With a median follow-up of 19 months (3–38+), three-year progression-free and overall survival were 6% and 31%, respectively.
Conclusion. The optimistic assumptions of this trial led to an early closure. However, the promising clinical benefit observed with the oral combination of lenalidomide and metronomic cyclophosphamide may justify its use for outpatient palliative treatment.
The standard treatment for patients with classical Hodgkin lymphoma (cHL) who relapse or progress after primary treatment consists of salvage chemotherapy followed by autologous hematopoietic stem cell transplantation (ASCT). This treatment strategy results in cure in 50–60% of patients [Citation1]. Unfortunately, patients who are refractory to salvage chemotherapy or relapse after ASCT have a median survival of two years, and rarely achieve durable CRs [Citation2]. Recently brentuximab vedotin have been approved by the US Food and Drug Administration for the treatment of cHL, but most patients benefit only marginally from them [Citation3]. Therefore, there is still a need for new therapies that are both active and tolerable in this heavily pretreated patient population.
The importance of cellular immunity in the regulation of cHL suggests that immunomodulatory agents might be active against this disease. Lenalidomide is an immunomodulatory drug with antiangiogenic and antineoplastic properties [Citation4,Citation5]. Lenalidomide reverses resistance to conventional chemotherapy in several B-cell-derived malignant cell lines [Citation6], and increased activity of cytotoxic T cells and NK cells by stimulating IL2 and gamma interferon production and inhibiting IL10 production [Citation7]. In addition, lenalidomide inhibits the migration and invasiveness of human endothelial cells via inhibition of VEGF production and Tie2/VEGF1 receptor activity [Citation8].
There is also evidence for the antiangiogenic and antitumor effects of continuous oral low-dose cyclophosphamide (metronomic doses) [Citation9,Citation10]. Unfortunately, only limited data are available on the efficacy of metronomic-dose cyclophosphamide —alone or in combination with other antiangiogenic agents— in cHL. A phase-II study evaluated the efficacy of the combination of metronomic-dose cyclophosphamide and vinblastine with rofecoxib in 50 patients with advanced malignancies (45 solid tumors and 5 lymphomas) [Citation11]. Three patients with cHL were enrolled and all three achieved a response (two complete and one partial responses), with a time to progression of 748, 418, and 749 days respectively.
Combining lenalidomide with metronomic-dose cyclophosphamide in cHL might have the advantage that, in addition to the antiangiogenic and antitumor activity of both drugs, the immunomodulatory effect of lenalidomide might enhance the efficacy of a metronomic dosing schedule. In a preclinical study by Blansfield et al. [Citation12], the combination of lenalidomide with metronomic-dose cyclophosphamide increased their respective antiangiogenic and antitumor activities.
Patients and methods
Eligible patients
Patients aged 18 years or older previously diagnosed with cHL according to the World Health Organization (WHO) classification [Citation13], who had progressed after ASCT or had shown disease progression or refractoriness to at least two prior lines of chemotherapy and were not considered eligible for ASCT were included. Patients who had previously undergone allogeneic transplantation were also eligible. The study (EUDRA CT no. 2009-016588-12) was conducted in compliance with the recommendations of the Declaration of Helsinki. It was approved by the Research Ethics Committees of all participating institutions and all patients signed informed consent.
Study design and treatment
A phase-II, open-label, multicenter, prospective, single treatment arm study was carried out. Study patients were treated in 28-day cycles. Cycle 1 (level 0) consisted of lenalidomide 20 mg/day for 21 days plus cyclophosphamide 50 mg/day on all 28 days of the cycle. Escalation of the lenalidomide dose to level 1 () was allowed in patients who did not experience any grade ≥ 3 hematologic toxicity or grade ≥ 2 non-hematologic toxicity during treatment cycle 1, and to level 2 in patients who received cycle 2 at dose level 1 and did not experience these toxicities. Patients who experienced grade 3–4 toxicity at any dose level received the next cycle at the next lower dose level. In patients who received lenalidomide at dose level − 1 or lower and experienced grade ≥ 3 hematologic toxicity, the cyclophosphamide dose was reduced to 50 mg q48h. Treatment was continued until disease progression or unacceptable adverse effects, for a maximum of 24 cycles.
Table I. Lenalidomide dose levels.
All adverse events (AEs) were assessed and coded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Assessment of response and statistical analysis
The primary endpoint was the overall objective response rate (ORR) as defined by the 1999 International Working Committee (IWC) criteria [Citation14]. Restaging computerized tomography scans were performed after Cycles 2, 4, and 7, and every three cycles thereafter. Clinical benefit was defined as complete remission (CR), partial remission (PR), or stable disease (SD) lasting longer than six months. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and safety assessment of the drug combination.
The sample size was calculated according to a Simon's two-stage design, with an alpha error of 0.025 and a beta error of 0.8. The activity of the drug combination would not be considered clinically significant if the ORR was lower than 40% (H0) and treatment would be considered promising if the ORR was higher than 60% (H1). Under these assumptions, 16 patients would be recruited for the first stage and, if at least seven objective responses were achieved (in the assessment after cycle 4), the study would proceed to the second stage. Failure to achieve seven responses would constitute a reason to halt the study because the efficacy would be below the expected range. In the second stage, recruitment would continue up to 46 patients. The coordinator of the study (A.R.) was the responsible for review the responses after the fourth cycle and decide the outcome of the trial.
Study results were analyzed by intention-to-treat, and all patients who initiated study treatment were considered evaluable for efficacy and toxicity. The correlation between two quantitative variables that did not conform to a Gaussian distribution was examined by calculating the Spearman's correlation coefficient. The χ2-test was used to measure the association between categorical variables in both groups. Time to progression or death was analyzed using the Kaplan-Meier method. The groups (according to categorical variables) were compared using the log-rank test. A 5% two-sided significance level was used for all tests.
Results
Patient characteristics
The study was stopped after 16 patients were enrolled because only five objective responses had been achieved (ORR 31%, 95% CI 11–58%) after treatment cycle 4. The patient characteristics are summarized in .
Table II. Patient characteristics.
The median number of prior therapies was five (range 2–6), 11 (71%) patients were refractory to the last prior therapy and 10 (68%) were primarily refractory. Four (25%) patients had not previously undergone ASCT: three because they were refractory to all previous therapies, and one patient aged 77 who was also refractory to second-line therapy (the last before enrollment). The 12 (75%) patients who underwent ASCT had progressed within a median of five months (range 3–23) after ASCT, and had received a median of two therapies after ASCT. Five (31%) patients had undergone allogeneic hematopoietic stem cell transplantation before study enrollment.
Treatment administration
A total of 110 cycles were administered to the 16 study patients. The median duration of treatment was seven cycles (range 1–24). The lenalidomide administered dose is shown in . The reasons for discontinuing treatment were as follows: disease progression in 12 (75%) patients, investigator's decision in one, patient's decision in one, and one toxic death. Only one (6%) patient completed the 24 protocol-planned treatment cycles.
Toxicity
Toxicity was recorded in 76% (84 of 110) of the lenalidomide-cyclophosphamide cycles. The recorded toxicity was severe (grade ≥ 3) in 43 (39%) cycles. The most common severe toxicities per cycle were as follows: neutropenia (14%), thrombocytopenia (7%), anemia (6%), lymphocytopenia (5%), and infections (4%).
Three patients (19%) experienced no grade > 2 toxicities. Fifty-six percent of patients experienced severe neutropenia in at least one treatment cycle. Severe thrombocytopenia, anemia, and lymphocytopenia occurred in 32%, 25%, and 19% of patients, respectively.
Special mention should be made to infections and liver toxicity. A total of 11 infectious events were noted in seven patients, of which five were severe (grade ≥ 3). Of these, three patients with grade 3 pneumonia and one patient with grade 3 bacteremia recovered fully. However, a non-neutropenic patient developed pneumonia that rapidly progressed to septic shock and respiratory failure that led to the patient's death. This event occurred after treatment cycle 5; the patient was in PR and had not experienced any other toxicity in the previous four cycles.
As regards to liver toxicity, one patient developed grade 3 cholestatic hepatitis and responded favorably to low-dose corticosteroids (< 10 mg/day). Another patient had two episodes of elevated transaminases (grades 3 and 4) and recovered after the lenalidomide dose was delayed and reduced. Finally, one patient developed grade 2 transaminitis that resolved spontaneously.
Efficacy and outcome
Overall, six patients (38%, 95% CI 15–64%) achieved objective response; five of them achieved partial response and one achieved complete response (and has maintained it to date). Five patients achieved SD lasting at least six months and five patients developed disease progression (one patient who was in CR after cycle 2 progressed before cycle 4). Ten patients (62%) achieved clinical benefit. Of the 11 patients who did not progress during the first four cycles, one died from toxicity in treatment month 5 and was considered not to have achieved clinical benefit despite being in PR.
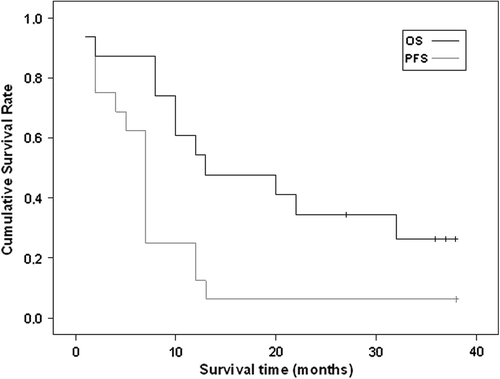
At the time of data analysis, 15 patients had progressed (one due to toxic death) and 11 had died (9 from disease progression, 1 from treatment toxicity, and 1 from toxicity of a subsequent therapy). Of the five living patients, two are free of disease (one without any additional treatment). The median time to progression was seven months (range 1–38+) and the median survival was 19 months (range 3–38+). With a median follow-up of 19 months (37 months for living patients), the three-year PFS and OS was 6% and 31%, respectively ().
After disease progression nine patients received brentuximab vedotin and three received everolimus (2 after brentuximab). Two of them achieved complete response with brentuximab vedotin and underwent allogeneic transplantation (one is still alive with no progression and one died from allogeneic transplantation toxicity), one is still alive and in partial response after brentuximab, and two are in partial response and receiving everolimus. The remaining patients died of lymphoma progression.
Discussion
When this study was being designed, the German Hodgkin Study Group (GHSG) reported the results of treatment with lenalidomide for 10 patients with cHL and two patients with lymphocyte-predominant HL, all heavily pretreated (median of 4 prior chemotherapies), within a compassionate-use (named-patient) program [Citation15]. The GHSG reported an ORR of 50% and no grade > 2 toxicity in this small group of patients. These striking results were used to define our study hypothesis, since the addition of cyclophosphamide might enhance not only the activity but also the toxicity. Therefore, an ORR lower than 60% would add nothing to lenalidomide monotherapy.
Two years later, Fehniger et al. [Citation16] reported the results of a phase-II multicenter study conducted in North America, which enrolled 38 heavily pretreated patients with cHL relapsed or refractory after ASCT. Lenalidomide was administered at 25 mg/day for 21 days in 28-day cycles. The intention-to-treat analysis yielded an ORR of 18% and a clinical benefit rate of 31%, with a median PFS of four months and a median OS of 20 months.
Despite the small number of patients enrolled, the results achieved by our study appear to be better than those reported by the North American study. The combination of lenalidomide with metronomic-dose cyclophosphamide achieved an ORR of 38% and a clinical benefit rate of 62%, with a median time to progression of seven months. As in the US study, a patient who was in PR after treatment cycle 4 achieved CR subsequently, completed the planned 24 cycles, and remains free of disease progression 38 months after study enrollment. We do not know whether this increase in activity is due to the addition of cyclophosphamide, to lenalidomide dose escalation (as allowed in our study), or to chance (due to the small number of patients enrolled).
Until a few years ago, the most active treatment for patients with cHL refractory or relapsed after ASCT was dose-adjusted chemotherapy with the GVD regimen (gemcitabine, vinorelbine, and liposomal adriamycin), which achieved an ORR of 75% and a CR rate of 17%, with a median survival of 3.5 years [Citation17]. However, it should be kept in mind that their study population had a better prognosis, 84% of patients were responsive to the last prior therapy (31% in our study) and the median number of prior therapies was three (5 in our study). Brentuximab vedotin have recently been approved by the U.S Food and Drug Administration for the treatment of these patients. In phase-II study that enrolled 102 patients (58% responsive to the last prior therapy, with a median of 3.5 prior therapies) [Citation3], an ORR of 75% and a CR rate of 34% was achieved with brentuximab vedotin; the median time to progression was 5.6 months, and, importantly, the median duration of CR was 20 months, with a significantly lower toxicity than that reported with GVD.
Bendamustine is another drug with significant activity in patients with cHL relapsed or refractory after ASCT. Bendamustine was examined at a dose of 120 mg/m² for two days (with granulocyte growth factor support) every three weeks in a phase-II study that enrolled 36 patients. ORR was 53%, CR rate was 33%, and median PFS was less than six months [Citation18].
In this setting, lenalidomide appears to be marginally active in patients with cHL relapsed or refractory after ASCT. However, the lenalidomide-cyclophosphamide combination achieved acceptable results (38% ORR, clinical benefit rate of 62%, median PFS of 7 months) even though the prognostic factors in our study population were more unfavorable that those of patients enrolled in the other studies reviewed (5 prior therapies, median progression after ASCT at 5 months, and 69% refractory to the last prior therapy).
The choice of treatment for cHL relapsed or refractory after ASCT depends on the goal of treatment and patient preference. In patients ineligible for allogeneic transplantation, the goal should be to prolong survival with good quality of life. In this scenario, brentuximab vedotin currently remains the drug of choice, but the lenalidomide-cyclophosphamide combination might be an acceptable option after failure to brentuximab if these early findings are confirmed in other studies with larger numbers of patients.
Declaration of interest: This study was funded by Celgene as an investigator-initiated trial. Dr. Rueda designed the research study and drafted the manuscript. All the authors contributed substantially to the acquisition, analysis or interpretation of the study data; performed research; and reviewed and approved the submitted and final versions of the manuscript. Drs. Rueda and Casanova have received research funding from Celgene. Drs. Rueda, García-Sanz and Casanova have received honoraria for participation in advisory boards, and/or speaker at meetings from Celgene. The others authors declare no conflict of interest.
References
- Josting A, Müller H, Borchmann P, Baars JW, Metzner B, Döhner H, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin's lymphoma. J Clin Oncol 2010; 28:5074–80.
- Moskowitz AJ, Perales MA, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol 2009;146:158–63.
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 2012;30:2183–9.
- Lu L, Payvandi F, Wu L, Hariri RJ, Man HW, Chen RS, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res 2009;77:78–86.
- Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 2008;140:36–45.
- Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood 2002;100:3063–7.
- Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD-8 + T cells in vitro. J Infect Dis 2003; 187:946–55.
- Zhang H, Vakil V, Braunstein M, Smith EL, Maroney J, Chen L, et al. Circulating endothelial progenitor cell in multiple myeloma: Implications and significance. Blood 2005;105:3286–94.
- Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nolè F, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: Antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 2002;13:73–80.
- Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, et al. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res 2002;62:2731–5.
- Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res 2006;12:3092–8.
- Blansfield JA, Caragacianu D, Alexander HR, Tangrea MA, Morita SY, Lorang D, et al. Combining agents that target the tumor microenvironment improves the efficacy of anticancer therapy. Clin Cancer Res 2008;14:270–80.
- Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissue. Geneva, Switzerland: World Health Organization; 2008.
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol 1999;17:1244–53.
- Boll B, Borchmann P, Topp MS, Hänel M, Reiners KS, Engert A, et al. Lenalidomide in patients with refractory or multiple relapsed Hodgkin lymphoma. Br J Haematol 2009;148:480–90.
- Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 2011;118:5119–25.
- Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol 2007;18:1071–9.
- Moskowitz AJ, Hamlin PA Jr, Perales MA, Gerecitano J, Horwitz SM, Matasar MJ, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol 2013;31:456–60.