Abstract
With the advent of multimodality therapy, the overall five-year survival rate from childhood cancer has improved considerably now exceeding 80% in developed European countries. This growing cohort of survivors, with many years of life ahead of them, has raised the necessity for knowledge concerning the risks of adverse long-term sequelae of the life-saving treatments in order to provide optimal screening and care and to identify and provide adequate interventions. Childhood cancer survivor cohorts in Europe. Considerable advantages exist to study late effects in individuals treated for childhood cancer in a European context, including the complementary advantages of large population-based cancer registries and the unrivalled opportunities to study lifetime risks, together with rich and detailed hospital-based cohorts which fill many of the gaps left by the large-scale population-based studies, such as sparse treatment information. Several large national cohorts have been established within Europe to study late effects in individuals treated for childhood cancer including the Nordic Adult Life after Childhood Cancer in Scandinavia study (ALiCCS), the British Childhood Cancer Survivor Study (BCCSS), the Dutch Childhood Oncology Group (DCOG) LATER study, and the Swiss Childhood Cancer Survivor Study (SCCSS). Furthermore, there are other large cohorts, which may eventually become national in scope including the French Childhood Cancer Survivor Study (FCCSS), the French Childhood Cancer Survivor Study for Leukaemia (LEA), and the Italian Study on off-therapy Childhood Cancer Survivors (OTR). In recent years significant steps have been taken to extend these national studies into a larger pan-European context through the establishment of two large consortia – PanCareSurFup and PanCareLIFE. The purpose of this paper is to present an overview of the current large, national and pan-European studies of late effects after childhood cancer. This overview will highlight the strong cooperation across Europe, in particular the EU-funded collaborative research projects PanCareSurFup and PanCareLIFE. Overall goal. The overall goal of these large cohort studies is to provide every European childhood cancer survivor with better care and better long-term health so that they reach their full potential, and to the degree possible, enjoy the same quality of life and opportunities as their peers.
“Paediatric cancer seen in perspective, then, puts us almost at the end of a long tunnel. Looking back in one direction towards the blackness of ‘no hope’ and ‘no cure’. Looking forward, we see a wide and open road with well-spaced way-stops en route to our final destination. Some of these way-stops surely are the development of ever more refined and precisely targeted methods of treatment, so that the increasing numbers of successfully treated children of today do not become the chronically ill adults of tomorrow.” This is how Professor Emeritus Giulio J. D’Angio described the ‘road of childhood cancer’ in his paper on pediatric cancer in perspective in 1975 with the end of the road being reached when childhood cancer is forever eliminated stressing that ‘indeed, cure is not enough’ [Citation1].
The treatment of childhood cancer is a success story of modern medicine, as effective regimens have been identified for many previously untreatable diseases. The survival rates from childhood cancer have improved at a remarkable pace over the past half century, with multidisciplinary, centralized treatment and high-quality supportive care. Survival rates have reached 80% at five years from diagnosis in developed European countries [Citation2]. It has been estimated that the number of survivors of cancer diagnosed during childhood and adolescence in Europe are between 300 000 and 500 000 with approximately one in every 640 young adults being a survivor of childhood cancer [Citation3]. The price of this remarkable success, however, has been high. To varying degrees, these former patients experience a wide spectrum of long-term adverse health consequences of the live-saving treatments that can affect almost any organ or body system [Citation4].
Unique possibilities exist to study late effects in a European setting including the complementary advantages of large population-based cancer registries with the oldest going back to the 1940s and 1950s together with rich and detailed hospital-based cohorts. Here we present both established and developing national survivor cohorts within Europe to study late effects in those treated as children for cancer highlighting the strong cooperation across Europe, in particular the EU-funded collaborative research projects PanCareSurFup and PanCareLIFE. Both projects have their origin in the PanCare Network (www.pancare.eu) inspired by the Erice Statement from 2007 [Citation5].
Childhood cancer survivor cohorts in Europe
This paper describes the established large national survivor cohorts, cohorts in the process of becoming established and national, and the two large European consortia totaling more than 100 000 former childhood cancer patients. We present an overview of studies of late effects focusing on differences and similarities in design and methods. Countries in Europe with established national childhood cancer survivor cohorts, countries in the process of establishing a national cohort, as well as countries being data providers to the two European collaborative studies are mapped in with detailed characteristics presented in . Details on study designs and methods and information on main research areas are presented in and .
Figure 1. Countries in Europe with established national childhood cancer survivor cohorts (black), those in the process of establishing a national cohort (dark gray), as well as countries being data providers to the two European collaborative studies (light gray).
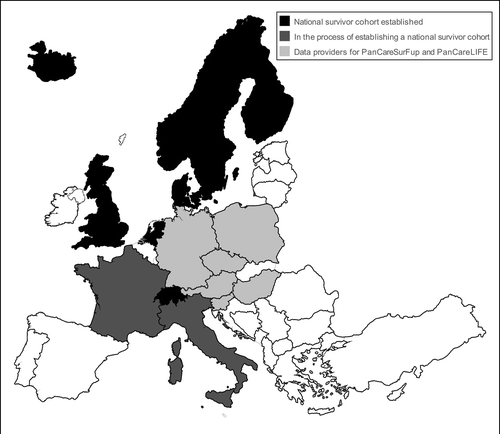
Table I. Characteristics of childhood cancer survivor cohorts in Europe.
Table II. Design and data collection as well as strengths and limitations of the European childhood cancer survivor cohort studies.
Table III. Overview of main research areas, ongoing and planned studies, and key publications.
Established national childhood cancer survivor cohorts
The Nordic Adult Life after Childhood Cancer in Scandinavia (ALiCCS)
The ALiCCS cohort comprises 33 160 children diagnosed with cancer below the age of 20; identification of children started at the establishment of the five national Nordic cancer registries in the 1940s and 1950s through 2008; children had to be at least one-year survivors. The ALiCCS cohort also includes 212 892 population comparison subjects randomly selected from Nordic civil registration systems.
Large-scale record linkage techniques provide accurate follow-up information on childhood cancer survivors, and enable comparisons with the general population to be accomplished. Such methods provide comprehensive knowledge of risks of treatment-induced late effects. Nationwide hospital registers in all five countries (Denmark, Finland, Norway, Iceland and Sweden) allow follow-up for a wide range of somatic late effects measured as medically verified diagnoses. In ongoing retrospective cohort studies, late effects in different organ systems are being studied. Morbidity among cohort members is analyzed on the basis of person-years at risk. Observed numbers of first-time hospitalizations for a broad range of sub-acute and chronic diseases in survivors are compared with that expected based on hospitalization rates derived from the population comparison cohort with relative and absolute risks estimated for specific chronic diseases. There has been linkage with several other registries for the ALiCCS study, allowing investigations for a broad variety of other outcomes, such as adverse events during pregnancy and birth as well as drug prescriptions.
Subsequent clinical case-cohort studies are designed to investigate associations, including dose-response effects, between specific elements of treatment regimens and the risk of late effects, and will include study subjects selected from the ALiCCS cohort including, however, only five-year survivors diagnosed from 1970. Cases are defined as those affected with a selected disease outcome (late effect) observed in the cohort studies. For cases and 600 sub-cohort members randomly selected from the cohort of five-year survivors representing cancer treatment in the entire five-year survivor cohort, information on cancer treatment is being abstracted from medical records, including drug names and cumulative doses as well as radiation field and doses. Organ dose reconstruction from pediatric radiation therapy including dosimetry for a variety of organs is being performed at the M.D. Anderson Cancer Center in Houston, USA [Citation6].
ALiCCS contributes data to PanCareSurFup and Denmark is contributing data to PanCareLIFE.
Our Nordic approach and choice of epidemiological designs allow systematic ascertainment of late effects in organ systems and will strengthen knowledge of all types of late sequelae that these patients encounter as they age. The main limitation is lack of treatment information; this aspect will be addressed in the clinical case-cohort studies.
The British Childhood Cancer Survivor Study (BCCSS)
The BCCSS cohort originally comprised 17 981 individuals diagnosed with childhood cancer before aged 15 years, between 1940 and 1991 in England, Wales or Scotland and who survived at least five years from diagnosis [Citation7]. The cohort has recently been extended to include an additional 16 509 individuals diagnosed between 1992 and 2006, but otherwise satisfying the criteria defining the original cohort.
Causes of deaths and incident cancers occurring subsequent to five-year survival are obtained through individual patient electronic record linkage with the national population-based death and cancer registries. Historically, between 2002 and 2007, a questionnaire was sent to 14 836 survivors aged over 16 years and 10 483 (71%) returned it completed. The questionnaire included several questions which had been included in the ‘General Household Survey’ which was undertaken on a probability sample of the national general population; these included frequency use of health services, educational attainment, occupational status, smoking, and alcohol consumption history. The extent to which survivors marry and produce liveborn offspring has also been compared with that expected from the national marriage and birth registries.
The BCCSS contributes data to PanCareSurFup.
Nested case-control studies have been undertaken to evaluate the association between increased cumulative exposure to different types of chemotherapy or radiotherapy and the increased risk of development of subsequent primary leukemia, bone cancer, soft tissue sarcoma, and central nervous system tumors.
The Dutch Childhood Oncology Group Program on long-term adverse effects after childhood cancer (DCOG LATER)
The DCOG LATER program is responsible for optimal care and research related to Dutch childhood cancer survivors. In recent years, the DCOG LATER group developed the DCOG LATER guidelines, set up seven outpatient clinics for survivors in The Netherlands, and designed the DCOG LATER study program consisting of two parts.
In the first part, all five-year survivors of childhood cancer treated in one of seven pediatric oncology centers between 1963 and 2002 for cancer before the age of 18 years have been identified and ascertained. The DCOG LATER nationwide hospital-based cohort consists of 6168 five-year survivors; baseline demographics about their first cancer diagnosis and treatment have been collected in a web-based database. In addition, a questionnaire focusing on risk factors, lifestyle, and health conditions was sent to the survivors in 2013 with questionnaire outcomes to be validated by information retrieved from general physicians and medical records. The first results of several observational studies on mortality, symptomatic cardiac disease, secondary primary malignancies and female reproductive function in the entire DCOG-LATER cohort are expected in 2015.
In the second part of the DCOG LATER study program, all 5545 living survivors will be invited for a visit to the research clinic for a physical examination, laboratory tests, diagnostic tests, and additional questionnaires. This clinical evaluation will provide a unique opportunity to validate the questionnaire outcomes and the results of linkage studies, to collect data on asymptomatic outcomes, and to gain insight in the additional value of screening tests. Furthermore, DNA collection will allow future analyses of genetic predisposition for specific treatment-related health problems. As all clinical data are collected within one visit, the burden for survivors is minimized substantially [Citation8]. The research project-specific questions within the projects are based on gaps in knowledge as identified during the establishment of the DCOG LATER guidelines for patient care. In 2007 the DCOG LATER VEVO study was initiated to evaluate female reproductive function in the DCOG LATER cohort. This study established the feasibility of similar studies in the Netherlands.
The DCOG LATER contributes data to both PanCareSurFup and PanCareLIFE.
The Swiss Childhood Cancer Survivor Study (SCCSS)
The Swiss Childhood Cancer Survivor Study (SCCSS) comprises all Swiss residents diagnosed with cancer before age 21 from 1976 until the present and registered in the Swiss Childhood Cancer Registry (SSCR), i.e. 7600 persons with 6100 still being alive and 4405 being five-year survivors. Depending on the outcome under study, different comparisons are made: with the general population, representative population surveys, and siblings.
Different methods are used to collect long-term outcome data: prospective data collection by hospitals during clinical follow-up visits; data linkages to Swiss routine statistics (population, birth and mortality registers, census data, hospital statistics); and questionnaire surveys to patients [Citation9].
Clinical follow-up is continued for 5–10 years after diagnosis in all nine Swiss pediatric cancer centers, and information on remissions, relapses, changes in therapy, and late effects is forwarded to the SCCR. Vital status, information about migration and current address are continuously updated via population registers and causes of death from Swiss mortality statistics. Observed mortality and cancer incidence are compared to that expected from the general population. Patient-reported outcomes on psychological and somatic morbidity, treatments, quality of life, health behaviors, follow-up care, and socio-economic outcomes are assessed in questionnaire surveys for all five-year survivors. The first questionnaires were mailed in 2007 and the first follow-up questionnaire has been sent to a sub-population. The content of the SCCSS questionnaire is similar to that of CCSS (the US Childhood Cancer Survivor Study) [Citation10] and BCCSS. The Swiss questionnaire is developed in different versions for three age groups (0–15, 16–19, > 19 years) and in three languages (French, German, and Italian). By December 2013, about 3500 patients diagnosed from 1976 to 2005 had received a questionnaire, and 72% had replied. Patients diagnosed between 2006 and 2010 will be contacted in 2015.
Focused studies (for subgroups of patients and controls defined by outcomes or treatments of interest and often in international collaborations) include detailed treatment information and validated outcomes from hospital charts and general practitioner notes. DNA collection is currently ongoing for sub-groups but is to be extended to the whole cohort.
The SCCSS study contributes data to both PanCareSurFup and PanCareLIFE.
The main limitation of SCCSS is the relatively small size reflecting the population size of Switzerland. Strengths include the inclusive representative design with an ongoing study of national coverage, involvement of all pediatric oncologists, a broad range of data collected in a standardized way thus allowing pooling with other studies.
Childhood cancer survivor cohorts developing towards becoming established and national
The French Childhood Cancer Survivor Study (FCCSS)
The FCCSS cohort is coordinated by the Centre for Research in Epidemiology and Population Health (CESP) of the National Institute for Health and Medical Research (INSERM) and aims to include about 18 000 individuals diagnosed with a malignant tumor (except leukemias), below the age of 20 years between 1946 and 1999 in France, and who survived at least five years from diagnosis. Today the cohort includes about 12 000 survivors. The establishment of a national cohort is expected in 2016.
In FCCSS, detailed treatment information is collected from medical records for all patients. Additionally, whole body dose reconstruction is planned for all patients who received radiation therapy, using software developed by INSERM CESP in collaboration with Gustav Roussy Institute [Citation11,Citation12]. Information on vital status and causes of deaths are obtained each year through individual patient electronic record linkage with the national population-based cause of death register. Between 2000 and 2010, a questionnaire was sent to the first sub-cohort of survivors (‘Euro2K’) including 3172 patients treated before 1986 in five centers, with a response rate of 76% among those still alive. Based on these survey data, investigations have been undertaken concerning the role of dose of specific type of chemotherapy and of radiation dose to specific bodily organs in subsequent primary neoplasms, early menopause, cardiovascular diseases, and diabetes.
In 2010, a biobank of constitutional DNA was set up, now including 2500 patients, and since 2014, yearly linkages to the national hospital database of France (linkage at individual level since 2006) has been performed to ascertain late effects.
The FCCSS study contributes data to PanCareSurFup. The Rhône-Alpes region (ARCERRA) is contributing data to PanCareLIFE.
The French Childhood Cancer Survivor Study for Leukaemia (LEA)
The LEA project aims to study determinants (medical, socio-economic, behavioral, and environmental) of medium- and long-term health and quality of life of patients treated for a childhood acute leukemia. The cohort includes patients with an acute leukemia diagnosis after January 1980, below the age of 19, surviving 2 years after diagnosis for myeloblastic acute leukemia patients or lymphoblastic acute leukemia patients grafted in first complete remission or four years after diagnosis for lymphoblastic acute leukemia patients not grafted in first complete remission. As of 31 October 2014, 3029 survivors constitute the cohort in the 14 participating French pediatric onco-hematology centers.
Information collected comprises medical data, relating to acute leukemia and physical late effects, and personal data, such as socioeconomic status, schooling, psycho-cognitive data or quality of life, for which age- and sex-matched population norms are available. Data are collected every two years until the patient is 20 years old and has had a 10-year follow-up duration from diagnosis or relapse. Thereafter, assessments are planned every four years. Studies in progress are focusing on sparsely explored physical late effects, such as thyroid tumors, metabolic syndrome, and fertility. The risk of sequelae in survivors of childhood standard-risk lymphoblastic acute leukemia is explored. The survivor's professional outcome is compared to that in general population. Risk factors relating to school performance are analyzed.
Molecular explorations are planned involving a whole-genome scan to identify variations associated with the risk of survivor's sequelae. In addition, the LEA study will use a nested case-control design to study the objective and subjective health of survivors, comparing survivors from the most disadvantaged backgrounds with those of other backgrounds for whom access to suitable examinations is facilitated by their socioeconomic status or geographic proximity to healthcare providers. Long-term outcome of healthy siblings (with a particular focus on hematopoietic stem cell donors) will be explored, as a control group for some sequelae.
The major strength of the LEA cohort is to undertake dedicated clinical examinations and laboratory exams to document the occurrence of late side effects. At present the participating pediatric onco-hematology centers cover nearly 75% of all French childhood leukemia survivors diagnosed since 1980. The ongoing extension of the cohort (inclusion of new pediatric onco-hematology centers and continuous inclusion of incident cases in the active centers) should ensure a higher representation.
The Italian Study on off-Therapy Childhood Cancer Survivors (OTR)
A national Italian cohort of childhood cancer survivors will be set up in the near future in Italy. The Off-Therapy Registry (OTR) of the Italian Association of Paediatric Haematology and Oncology (AIEOP) originally started up in 1980 enrolling children with cancer prospectively when they had completed therapy. At the start of this register prevalent cases were also included with the first patient being diagnosed in 1960. Since 1987, a more systematic enrolment of patients from the clinical centers was established. Today, the clinical OTR register is almost complete for children below 15 (92% recruitment rate of expected cases), whereas the register is less complete for those aged 15–19 years (covers only around 25% of all cases). Detailed information on treatment is available mainly from abstraction of medical records.
The clinical OTR register will be merged with data on children diagnosed with cancer from provincial and regional cancer registries (AIRTUM) with complete registration of all cancers within that specific geographical area [Citation13]. The AIRTUM registries cover cancer registration in about 50% of the Italian population.
An agreement between the AIEOP network and AIRTUM about pooling data will allow the establishment of a large national Italian cohort of childhood cancer survivors that become eligible at the off-therapy stage. Nationwide hospital- and regional cancer register-based data will be entered into an updated version of the original OTR register. The register will include more than 30 000 survivors covering more than 95% of all childhood cancer survivors in Italy. Based on record linkage to national health data (combined data from regional hospital registries), the aim of this updated register is to study cause-specific hospitalizations, second primary cancers, and mortality.
Both PanCareSurFup and PanCareLIFE receive data from Italian population-based registries and from hospital-based records.
The EU-funded collaborative research projects
PanCare Childhood and Adolescent Cancer Survivor Care and Follow-Up Studies (PanCareSurFup)
PanCareSurFup is a five-year project that started in 2011.The PanCareSurFup cohort comprises more than 100 000 children and adolescents diagnosed with cancer below the age of 21 from 1940s to 2000s identified in both national and hospital-based registries in the Nordic countries, Slovenia, UK, France, the Netherlands, Hungary, Switzerland, and Italy. The project aims to establish a single retrospective pan-European cohort of long-term survivors of childhood and adolescent cancer with systematic ascertainment of at least one of the following endpoints: cardiac disease, subsequent primary neoplasms, and late mortality. From this cohort a virtual pan-European database will be constructed storing characteristics of these patients as available in individual databases. Currently case-control studies are being undertaken to explore the relationship between previous cancer therapy and the risk of development of subsequent primary neoplasms and serious cardiac outcomes. Ascertainment of these cases will be done by questionnaire, by linkage to hospital registries, or by clinical visit, depending on the data provider. Besides the partners named in the project, some of whom are data providers, a number of other institutions will also provide data to the PanCareSurFup cohort and case-control studies (see Acknowledgments).
An estimated number of 600 cardiac cases are expected including survivors with heart failure, cardiac ischemia, pericarditis, valvular disease, and arrhythmia. For the subsequent primary neoplasm studies, focus will be on bone cancer, soft-tissue sarcoma, digestive, and genitourinary carcinomas with an estimated number of 300 cases in each of these four studies and a similar number of matched controls. For all cases and controls, information on cancer treatment is being abstracted from medical records, including cumulative drug doses and radiation field and doses. Radiotherapy organ dose reconstruction will be performed at the Institut Gustave Roussy in France [Citation11,Citation12].
Strengths include long-term follow-up in high quality registries together with detailed treatment information from medical record abstraction and organ dosimetry. A potential limitation relates to data being provided from a mixture of population- and hospital-based registries. However, a large number of individuals is included in both the underlying cohorts and in the case-control studies and will provide the largest ever studies of the relevant outcomes.
PanCareSurFup has also identified a need for pan-European guidelines for long-term follow-up of childhood cancer survivors [Citation14]. The information obtained about the risks factors for serious cardiac disease and subsequent primary neoplasms will be incorporated into evidence-based guidelines by PanCareSurFup.
PanCareLIFE
PanCareLIFE is a five-year project that started in 2013. PanCareLIFE studies the risks of impairments in female fertility, in hearing following cisplatin therapy, and in quality of life, both independently and in relation to impairments in fertility and hearing. Additionally, biospecimens will be collected for related genetic studies. PanCareLIFE comprises a number of different studies with non-overlapping patient and survivor populations. Besides the partners named in the project, some of whom are data providers, a number of other institutions will also provide data for PanCareLIFE's studies (see Acknowledgments).
PanCareLIFE's research team includes investigators from eight European countries who will enrol as many as 12 000 well-characterized research subjects into observational studies and molecular genetic investigations to identify risk factors, both genetic and non-genetic, linked to decrements in fertility and ototoxicity. Quality-of-life studies will evaluate the impact of fertility and ototoxicity. PanCareLIFE will advance the state-of-the-art in survivorship studies by evaluation of large cohorts with observational and genetic tools that will provide better knowledge of individual risk factors. Survivors can then be stratified into groups benefitting from personalized, evidence-based care. Information from PanCareLIFE studies will be incorporated into new guidelines for fertility preservation and will disseminate the results from this project widely to clinicians, scientists, and survivors and their families.
Discussion
A growing number of large national childhood cancer survivor cohorts have been established in Europe over recent decades evaluating a broad range of late effects. Other cohorts are being developed with the goal of becoming established and national. Several of these cohorts take advantage of large population-based cancer registries with the oldest registries in Europe going back to the 1940s providing unrivalled opportunities to study lifetime risks. By systematic ascertainment of organ dysfunctions and subsequent primary neoplasms, these studies will provide information on all types of late effects with estimations of relative and absolute risks for specific disorders. Furthermore, information on the cumulative risk for childhood cancer survivors of being diagnosed with a subsequent primary neoplasm or hospitalized for a somatic disease will be provided in different phases of life compared to that in the general population. As study subjects are identified from large population-based registries, access to information on all patients and comparisons with virtually complete follow-up of study populations will be available. Thus, such data are less susceptible to participation and response biases. As information on disease outcomes will be obtained from health registries and not from self-reports, data will not be influenced by recall or reporting bias.
Using the unique resources in several of the European countries for conducting epidemiological research by linkage to a variety of different population and health registries going long back in time will further strengthen the knowledge of both secondary carcinogenesis and risk of organ dysfunction that these patients encounter as they age, filling out the gaps in knowledge about very late disease outcomes that emerge up to and beyond the age of 60. Although population- and register-based retrospective cohort studies will constitute a comprehensive, powerful surveillance instrument for estimating risks for medically verified diseases in survivors, the main limitation of such studies, however, might be lack of treatment information – a limitation that will be addressed in clinical case-cohort or case-control studies including the collection of detailed information on all past cancer treatments.
In several of the cohort studies, a ‘gold standard’ approach for exposure assessment is implemented, in which organ dose reconstruction from radiation therapy and administrated chemotherapy received will be quantified from medical records and radiotherapy schemes and related to late effects. Computation of individual organ radiation doses and drug doses will make it possible to interpret the epidemiological results in the light of dose-response evaluations.
Cohort studies with study subjects and outcomes based solely on population-based registries are limited by the information available in these administrative health registries. Some of the register-based cohorts and those recruiting patients at the pediatric oncological wards, however, have the possibility to define data needed for ongoing and future studies and to obtain high quality data from thorough clinical examinations of survivors. Additional data on health behaviors, such as smoking, diet, and physical activity that may modify the risks associated with treatment [Citation4] can be obtained in some studies, and several are able to store biosamples to test future hypotheses as they emerge.
Within Europe complementing the large-scale cohort studies using population-based registries are the well-established hospital-based cohort studies which benefit from detailed information measured on individual patients which is rarely available to population-based cohort studies. This detailed information includes: comprehensive treatment information, genotypic information from blood or saliva samples, and outcomes of clinical tests which need to be undertaken in a clinic setting.
In recent years great strides have been taken to coordinate efforts to exploit these advantages in the European context through the establishment of two large EU-funded collaborative research projects by investigators from several European countries focusing on cardiac toxicity, subsequent primary neoplasms, and late mortality (PanCareSurFup) and female infertility, cisplatin-induced ototoxicity, and quality of life (PanCareLIFE). These two large consortia have several advantages including very large cohort studies combining both register- and hospital-based data with data from surveys, clinical case-control studies with detailed treatment information and dosimetry evaluation, as well as genetic evaluations.
New survivor cohorts are emerging and some of these cohorts are already contributing data to the European collaborative studies. As an example of a new cohort that is based in a cancer registry, the VIVE study was developed out of the German Childhood Cancer Registry. The first survey of the VIVE cohort is on-going and will be finalised in 2015 (Principal Investigator: Dr. G. Calaminus, Münster).
The therapeutic challenge of today is to reduce the treatment-related complications, while maintaining the high survival rate. To fully understand the health risks resulting from treatment and to adjust future treatment regimens, the first step is to characterize the morbidity among survivors and to identify those with the highest risk for late effects [Citation4]. Complementary advantages of ongoing European studies using different designs and methodologies will all contribute to important new knowledge about late effects. Published findings on late effects all stress that childhood cancer survivors need tailored follow-up care to identify health problems after treatment at an early stage. Furthermore, coordination of complex care is needed. Thus pediatric oncologists and other healthcare providers need to work together to achieve optimal models of care.
A risk-stratified approach to health surveillance was proposed by the Scottish Intercollegiate Guidelines Network in 2004 identifying three different groups of survivors who required an increasing intensity of follow-up [Citation15]. Since then, several groups in Europe and the US have developed guidelines [Citation16]. In 2010 the guideline groups started the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) (www.ighg.org) with the overall aim of establishing a common vision and integrated strategy for the surveillance of chronic health problems and subsequent primary neoplasms in childhood, adolescent, and young adult cancer survivors and to reduce duplication of effort, optimize the quality of care, and improve quality of life for this group of survivors [Citation16]. Recently, the first set of guidelines for breast cancer and cardiomyopathy surveillance from this worldwide collaboration have been published, with substantial input from Work Package (WP) 6 in PanCareSurFup [Citation17,Citation18]. PanCareSurFup and IGHG have also collaborated in the development of surveillance guidelines for female and male gonadotoxicity (manuscripts in preparation), and are currently working on guidelines for secondary thyroid cancer and central nervous system neoplasms. This collaboration will continue to develop surveillance guidelines for several other important and large topics, including metabolic syndrome, vasculopathy, and growth hormone deficiency, whilst WP 6 will develop guidelines for many other smaller topics (e.g. dental, hepatic). In addition, responding to a recent European survey showing great variability in the provision of long-term follow-up [Citation19], WP 6 is developing evidence-based guidelines for the delivery of such follow-up, including the important issues of age-appropriate transition of care from pediatric to adult services, models by which long-term follow-up care can be provided effectively, and the provision of health promotion information for survivors and their families. A recent PanCare survey [Citation20] highlighted the urgent need for fertility preservation guidelines, which is one of the major elements of the PanCareLIFE study.
The European Network for research on Cancer in Children and Adolescents (ENCCA) is another European project working in parallel with PanCareSurFup and PanCareLIFE. The Survivorship Passport is being developed within ENCCA and is intended to be an electronic tool containing comprehensive information on the medical history of each patient ending cancer therapy. The passport includes recommendations for tailored follow-up based on up-to-date clinical guidelines developed with PanCareSurFup and IGHG to facilitate the prevention, early detection and treatment of potential late effects or relapses. The passport is generated through a secured web-based platform being patient oriented, accessible in multiple languages by patients, and clinicians among others and can be integrated with national, hospital, and clinical trials databases. It will also help in making survivors, general practitioners, and healthcare professionals aware of the potential risks or late effects stemming from the previous disease and treatment received.
The overall goal of these large survivor cohorts and collaborative studies in Europe is to provide every European childhood cancer survivor with better care and better long-term health so that they reach their full potential, and to the degree possible, enjoy the same quality of life and opportunities as their peers.
Supplementary material available online
Supplementary Acknowledgments available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1008648.
ionc_a_1008648_sm3913.pdf
Download PDF (55.5 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- D’Angio GJ. Pediatric cancer in perspective: Cure is not enough. Cancer 1975;35(3 Suppl):866–70.
- Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer 2009;45:992–1005.
- Hewitt M, Weiner SL, Simone JV, editors. Childhood cancer survivorship: Improving care and quality of life. Washington, DC: National Academy of Sciences; 2003.
- Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70.
- Haupt R, Spinetta JJ, Ban I, Barr RD, Beck JD, Byrne J, et al. Long term survivors of childhood cancer: Cure and care. The Erice statement. Eur J Cancer 2007;43: 1778–80.
- Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res 2006;166:141–57.
- Hawkins MM, Lancashire ER, Winter DL, Frobisher C, Reulen RC, Taylor AJ, et al. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer 2008;50:1018–25.
- Overbeek A, van den Berg MH, Kremer LC, van den Heuvel-Eibrink MM, Tissing WJ, Loonen JJ, et al. A nationwide study on reproductive function, ovarian reserve, and risk of premature menopause in female survivors of childhood cancer: Design and methodological challenges. BMC Cancer 2012;12:363.
- Kuehni CE, Rueegg CS, Michel G, Rebholz CE, Strippoli MP, Niggli FK, et al. Cohort profile: The Swiss childhood cancer survivor study. Int J Epidemiol 2012; 41:1553–64.
- Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol 2002;38:229–39.
- Alziar I, Bonniaud G, Couanet D, Ruaud JB, Vicente C, Giordana G, et al. Individual radiation therapy patient whole-body phantoms for peripheral dose evaluations: Method and specific software. Phys Med Biol 2009;54:N375–83.
- Benadjaoud MA, Bezin J, Veres A, Lefkopoulos D, Chavaudra J, Bridier A, et al. A multi-plane source model for out-of-field head scatter dose calculations in external beam photon therapy. Phys Med Biol 2012;57:7725–39.
- Italian cancer figures, report 2012: Cancer in children and adolescents. Epidemiol Prev 2013;37(1 Suppl 1):1–225.
- Brown MC, Levitt GA, Frey E, Bardi E, Haupt R, Hjorth L, et al. The views of European clinicians on guidelines for long-term follow-up of childhood cancer survivors. Pediatr Blood Cancer Epub 2014 Nov 8.
- Scottish Intercollegiate Guidelines Network. Long term follow up of survivors of childhood cancer. A national clinical guideline. Edinburgh, Scotland: Healthcare Improvement, Scotland; 2004.
- Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 2013;60:543–9.
- Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol Forthcoming 2014.
- Mulder RL, Kremer LC, Hudson MM, Bhatia S, Landier W, Levitt G, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2013;14:e621–9.
- Essig S, Skinner R, von der Weid NX, Kuehni CE, Michel G. Follow-up programs for childhood cancer survivors in Europe: A questionnaire survey. PLoS One 2012;7:e53201.
- Terenziani M, Spinelli M, Jankovic M, Bardi E, Hjorth L, Haupt R, et al. Practices of pediatric oncology and hematology providers regarding fertility issues: A European survey. Pediatr Blood Cancer 2014;61:2054–8.
- de Fine Licht S, Winther JF, Gudmundsdottir T, Holmqvist AS, Bonnesen TG, Asdahl PH, et al. Hospital contacts for endocrine disorders in Adult Life after Childhood Cancer in Scandinavia (ALiCCS): A population-based cohort study. Lancet 2014;383:1981–9.
- Holmqvist AS, Olsen JH, Andersen KK, de Fine Licht S, Hjorth L, Garwicz S, et al. Adult life after childhood cancer in Scandinavia: Diabetes mellitus following treatment for cancer in childhood. Eur J Cancer 2014;50:1169–75.
- Rebholz CE, Reulen RC, Toogood AA, Frobisher C, Lancashire ER, Winter DL, et al. Health care use of long-term survivors of childhood cancer: The British Childhood Cancer Survivor Study. J Clin Oncol 2011;29:4181–8.
- Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010; 304:172–9.
- Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 2011; 305:2311–9.
- Overbeek A, van den Berg MH, Hukkelhoven CW, Kremer LC, van den Heuvel-Eibrink MM, Tissing WJ, et al. Validity of self-reported data on pregnancies for childhood cancer survivors: A comparison with data from a nationwide population-based registry. Hum Reprod 2013;28:819–27.
- Sieswerda E, Postma A, van Dalen EC, van der Pal HJ, Tissing WJ, Rammeloo LA, et al. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol 2012; 23:2191–8.
- van den Berg M, van Dulmen-den BE, Overbeek A, Ronckers C, van DW, Kremer L, et al. Fertility studies in female childhood cancer survivors: Selecting appropriate comparison groups. Reprod Biomed Online 2014;29:352–61.
- Michel G, Rebholz CE, von der Weid NX, Bergstraesser E, Kuehni CE. Psychological distress in adult survivors of childhood cancer: The Swiss Childhood Cancer Survivor study. J Clin Oncol 2010;28:1740–8.
- Rebholz CE, Rueegg CS, Michel G, Ammann RA, von der Weid NX, Kuehni CE, et al. Clustering of health behaviours in adult survivors of childhood cancer and the general population. Br J Cancer 2012;107:234–42.
- Wengenroth L, Rueegg CS, Michel G, Essig S, Ammann RA, Bergstraesser E, et al. Life partnerships in childhood cancer survivors, their siblings, and the general population. Pediatr Blood Cancer 2014;61:538–45.
- de Vathaire F, El-Fayech C, Ben Ayed FF, Haddy N, Guibout C, Winter D, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: A retrospective cohort study. Lancet Oncol 2012;13: 1002–10.
- Schwartz B, Benadjaoud MA, Clero E, Haddy N, El-Fayech C, Guibout C, et al. Risk of second bone sarcoma following childhood cancer: Role of radiation therapy treatment. Radiat Environ Biophys 2014;53:381–90.
- Thomas-Teinturier C, El Fayech C, Oberlin O, Pacquement H, Haddy N, Labbe M, et al. Age at menopause and its influencing factors in a cohort of survivors of childhood cancer: Earlier but rarely premature. Hum Reprod 2013;28:488–95.
- Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010;28:1308–15.
- Berbis J, Michel G, Baruchel A, Bertrand Y, Chastagner P, Demeocq F, et al. Cohort Profile: The French Childhood Cancer Survivor Study For Leukaemia (LEA Cohort). Int J Epidemiol Epub 2014 Mar 17.
- Berbis J, Oudin C, Alessandrini M, Vercasson C, Barlogis V, Chambost H, et al. Quality of life in minor siblings of childhood leukemia survivors, long-term after diagnosis: A LEA study (for Leucemies de l’Enfant et de l’Adolescent-childhood and adolescent leukemia). Psychooncology Epub 2014 Oct 28.
- Le Meignen M, Auquier P, Barlogis V, Sirvent N, Contet A, Simeoni MC, et al. Bone mineral density in adult survivors of childhood acute leukemia: Impact of hematopoietic stem cell transplantation and other treatment modalities. Blood 2011;118:1481–9.
- Oudin C, Simeoni MC, Sirvent N, Contet A, Begu-Le CA, Bordigoni P, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood 2011;117:4442–8.
- Ceschel S, Casotto V, Valsecchi MG, Tamaro P, Jankovic M, Hanau G, et al. Survival after relapse in children with solid tumors: A follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer 2006;47:560–6.
- Haupt R, Valsecchi MG, Silvestri D, De LP, Napoli S, Masera G, et al. Early and late deaths after elective end of therapies for childhood cancer in Italy. Int J Cancer 2000;86:393–8.
- Pivetta E, Maule MM, Pisani P, Zugna D, Haupt R, Jankovic M, et al. Marriage and parenthood among childhood cancer survivors: A report from the Italian AIEOP Off-Therapy Registry. Haematologica 2011;96:744–51.
