Abstract
Background. In recent years, evidence supporting multimodality treatment for oesophageal, oesophagogastric junction (OGJ), and gastric cancer has accumulated. This population-based cohort-study investigates trends and predictors of utilisation of multimodality treatment for oesophagogastric cancer in the Netherlands.
Patients and methods. Data were obtained from the Netherlands Cancer Registry regarding patients with oesophageal (n = 5450), OGJ (n = 2168) and gastric cancer (n = 6683) without distant metastases who had undergone R0 or R1 surgery diagnosed between 2000 and 2012. Follow-up was completed until February 2014. Preoperative/postoperative chemotherapy and/or radiotherapy combined with surgery were considered multimodality treatment. Logistic regression analysis was performed to analyse the association of age, gender, socioeconomic status, clinical T and N classification, hospital type, comprehensive cancer centre network region, and year of diagnosis, with multimodality treatment receipt. Additional analyses were performed to explore differences in trends of utilisation of multimodality treatment between academic and non-academic hospitals.
Results. Multimodality treatment utilisation for oesophageal, OGJ and gastric cancer increased significantly to 90%, 85% and 56% in 2012, respectively. In oesophageal and OGJ cancer patients, preoperative chemoradiotherapy was most frequently administered (85% and 47% in 2012, respectively), and in gastric cancer patients preoperative chemotherapy (47% in 2012). Lower age, higher clinical T and N classification, and diagnosis in more recent years were significantly associated with more frequent multimodality treatment receipt. The adoption of most types of multimodality treatment in academic hospitals preceded non-academic hospitals by a year.
Conclusion. In the Netherlands, the utilisation of multimodality treatment for oesophagogastric cancer has significantly increased during the past decade, especially in oesophageal and OGJ cancer. Multimodality treatment utilisation was especially dependent on patient and tumour characteristics and year of diagnosis, but multimodality treatment trends seem to be related to the publication of landmark studies, participation in nationally running clinical trials, and hospital type, preceding national guidelines.
The incidence of oesophageal, oesophagogastric junction (OGJ), and gastric cancer is relatively low in the Netherlands (population approx. 16 million). Around 2600 and 1500 patients were newly diagnosed with oesophageal/OGJ and gastric cancer in 2012, respectively [Citation1]. Surgery has a central role in the potentially curative treatment for oesophagogastric cancer, but despite optimisation of surgical quality [Citation2,Citation3], overall survival (OS) remains poor [Citation4] with five-year overall survival rates of 13% for oesophageal and 19% for gastric cancer in the Netherlands [Citation1]. In recent years, evidence supporting multimodality treatment for oesophageal, OGJ and gastric cancer in patients selected for clinical trials has accumulated [Citation5–7].
The results of the Intergroup 0116 trial, published in 2001, showed an absolute survival benefit of 17% on five-year OS for gastric cancer patients who received postoperative chemoradiotherapy (CRT) [Citation7]. The results of the MAGIC trial published in 2006, demonstrated that patients with gastric or OGJ cancer randomised for perioperative chemotherapy (CT) had a significantly improved five-year OS (absolute benefit of 13%) [Citation6]. More recently, results from the CROSS trial published in 2012 showed that the addition of preoperative CRT in oesophageal and OGJ cancer patients improved five-year OS by an absolute 13% [Citation5]. These results led to the implementation of multimodality treatment in the national guidelines of the Netherlands; for gastric cancer in May 2009 recommending perioperative CT and for oesophageal cancer in December 2010 recommending preoperative CRT. In the Netherlands, no separate national guideline exists for OGJ cancer, but the guidelines for oesophageal (2010) and gastric cancer (2009) both consider OGJ cancer and recommend preoperative CRT and perioperative CT, respectively.
It is unknown to what extent the results of randomised clinical trials correspond to daily clinical practice. Obviously, the results of randomised clinical trials are based upon selected patients, whereas patients in daily clinical practice are unselected. Also, not all unselected oesophagogastric cancer patients are eligible for the evidence-based treatment [Citation8,Citation9]. Consequently, the effect of an evidence-based treatment may differ in a nationwide population. The first purpose of this Dutch population-based cohort-study was to identify trends in the utilisation of multimodality treatment for non-metastasised oesophageal, OGJ, and gastric cancer in in the Netherlands between 2000 and 2012. The second purpose was to investigate factors associated with the utilisation of multimodality treatment. The third purpose was to explore differences in trends of utilisation of multimodality treatment between academic and non-academic hospitals.
Patients and methods
The Netherlands Cancer Registry
Data were obtained from the Netherlands Cancer Registry (NCR), wherefore trained registrars routinely collect information on all newly diagnosed malignancies in the Netherlands 9–12 months after diagnosis from hospital records. Quality of the data are high and completeness is estimated to exceed 95% [Citation10]. The NCR belongs to the comprehensive cancer centre (CCC) which was formerly divided into nine regional comprehensive cancer networks. The CCC is also responsible for among others the organisation of oncology consultation by the academic hospitals for the non-academic hospitals. In the NCR, topography and morphology are coded according to the International Classification of Diseases for Oncology (ICD-O). Tumours are staged according to the International Union Against Cancer tumour node metastasis (TNM) classification in use at the year of diagnosis. For the purpose of this study the clinical and pathologic TNM classifications from before 2010 were all converted according to the seventh edition [Citation11]. In the NCR, vital status was obtained from the nationwide population registries network, which provides complete coverage of all Dutch citizens. Follow-up was completed until the first of February 2014. The NCR Review Board approved the study.
Patient selection, inclusion and exclusion
All patients diagnosed in the Netherlands between January 2000 and December 2012 with invasive oesophageal (C15.1–15.9), OGJ (C16.0), and gastric cancer (C16.1–16.9) were selected from the NCR (), thereby not selecting patients with cervical oesophageal cancer (C15.0) and with non-epithelial cancers. Patients were included if they were eligible for treatment with curative intent, defined as no signs of metastatic disease at time of diagnosis and at surgery, i.e. cM0 and pM0 according to the seventh TNM classification. To secure similar study populations, patients were excluded in case of no surgery, palliative or macroscopically incomplete (R2) resection, as they were not eligible for postoperative treatment with curative intent. Patients operated outside the Netherlands were excluded (0.2%).
Figure 1. Schematic diagram of patient selection, inclusion and exclusion. The numbers between brackets represent the total number of patients. cM0, clinically M0 according the TNM classification; ICD-O C, International Classification of Diseases for Oncology codes; pM0, pathologically M0 according the TNM classification; R0, microscopically complete resection; R1, microscopically incomplete resection; R2, macroscopically incomplete resection. * According to the 7th version of the TNM classification.
Multimodality treatment and predictors
Data on treatment were also obtained from the NCR. Distinction was made between chemotherapy, radiotherapy and concurrent chemoradiotherapy. All treatments combined with surgery were considered multimodality treatment. Age was categorised into four age groups (< 45, 45–59, 60–74 and 75 or older). Socioeconomic status was categorised into quartiles according to the socioeconomic status of the four-digit postal code area as calculated by Statistic Netherlands, which is based on a combination of factors including income and the average price of houses. Eight university hospitals and one dedicated oncology hospital were categorised as academic and all other hospitals as non-academic. Patients were classified to academic and non-academic according to the hospital where they had undergone surgery, as hospital of chemotherapy or radiotherapy was not applicable to all patients. If the hospital of surgery was not registered, patients were classified according to the hospital where the chemotherapy and/or radiotherapy was administered. Region was according to the nine former CCC networks.
Statistical analysis
The utilisation of multimodality treatment was measured by receipt and type. Multimodality treatment receipt was calculated as the percentage of patients who received multimodality treatment in reference to the total study population. To analyse trends in the utilisation of multimodality treatment, percentages of multimodality treatment receipt were calculated per type of multimodality treatment per year. To analyse factors associated with utilisation of multimodality treatment, multivariable logistic regression analysis was performed with multimodality treatment receipt as outcome. The following factors were investigated: age, gender, socioeconomic status, clinical T and N classification, hospital type, CCC-region, and year of diagnosis. Additional analyses were performed to explore differences in trends of utilisation of multimodality treatment between academic and non- academic hospitals. For that purpose, the percentage of patients that received multimodality treatment per type of multimodality treatment per year was calculated for academic and non-academic hospitals separately. Based upon the observed trends for academic and non-academic hospitals, two logistic regression analyses were performed with multimodality treatment receipt as outcome using data of two different time periods, 2000–2007 and 2008–2012, adjusting for the abovementioned factors. For comparison purposes all preoperative and postoperative chemotherapy and/or radiotherapy treatments were combined as multimodality treatment in the multivariable logistic regression analysis. All statistical analyses were performed using STATA.
Results
The study population consisted of patients with cpM0 oesophageal (5445), OGJ (2163), or gastric cancer (6666) who had undergone an R0-1 resection (). Most patients had a clinical T2-4 tumour classification at diagnosis. Up to 48% of patients had clinically lymph node positive disease (). All nine academic hospitals operated patients with oesophageal, OGJ, and gastric cancer yearly until 2011, when one academic hospital stopped performing this type of surgery. Oesophageal cancer patients were operated in 31 non-academic hospitals in 2000, which decreased to 15 in 2012, OGJ cancer patients in 31, which decreased to 15, and gastric cancer patients in 78, which decreased to 43.
Table I. Patient and treatment characteristics of non-metastasised oesophageal, oesophagogastric junction and gastric cancer patients who had undergone an R0-1 resection diagnosed between 2000 and 2012 in the Netherlands.
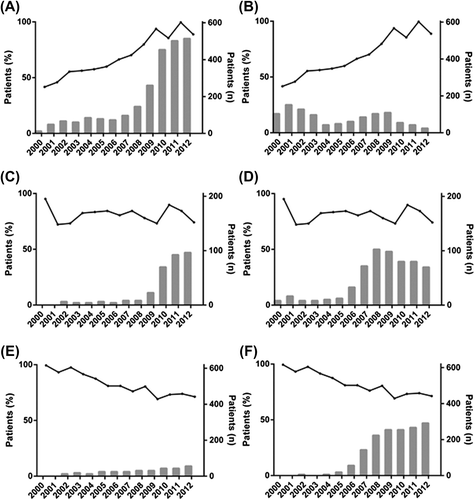
The percentage of patients who received multimodality treatment for oesophageal, OGJ, and gastric cancer increased from 20%, 6% and 1% in 2000 to 90%, 85% and 56% in 2012, respectively (). In oesophageal cancer, preoperative CRT was most frequently administered, up to 85% in 2012 after a rapid increase since 2008 (). The utilisation of preoperative CT with or without postoperative CT for oesophageal cancer was more limited than preoperative CRT (). In OGJ cancer patients, preoperative CRT was administered up to 47% in 2012 after a rapid increase since 2009 (). In this group, the administration of preoperative CT with or without postoperative CT was the highest in 2008 with 50%, but its use decreased thereafter to 34% in 2012 (). In gastric cancer patients, postoperative CRT (with or without preoperative CT) was very moderately administered up to 9% in 2012 (). The use of preoperative CT with or without postoperative CT for gastric cancer was higher, up to 47% in 2012 after a rapid increase since 2006 ().
Figure 2. Utilisation of multimodality treatment for cpM0/R0-1 oesophagogastric cancer patients in the Netherlands. For oesophageal cancer patients preoperative chemoradiotherapy (A) and preoperative chemotherapy with or without postoperative chemotherapy (B). For oesophagogastric junction cancer patients preoperative chemoradiotherapy (C) and preoperative chemotherapy with or without postoperative chemotherapy (D). For gastric cancer patients postoperative chemoradiotherapy with or without preoperative chemotherapy (E) and preoperative chemotherapy with or without postoperative chemotherapy (F). The bars depict the percentage of patients that received preoperative chemotherapy (plotted on the left y-axis), and the lines depict the total number of operated patients (plotted on the right y-axis).
For oesophageal, OGJ and gastric cancer patients, higher age and several CCC-regions were significantly associated with less multimodality treatment utilisation (). For all cancer types, higher clinical tumour classification, clinically lymph node positive disease, and diagnosis in more recent years were significantly associated with a higher chance of multimodality treatment receipt. For gastric cancer, a higher socioeconomic status was associated with more multimodality treatment receipt. For OGJ cancer, several CCC-regions were associated with more multimodality treatment receipt. Gender and hospital type were not significantly associated with the utilisation of multimodality treatment.
Table II. Multivariable logistic regression analysis to investigate the association of factors with multimodality treatment receipt for non-metastasised oesophageal, oesophagogastric junction and gastric cancer patients who had undergone an R0-1 resection diagnosed between 2000 and 2012 in the Netherlands.
Additional analysis showed that in academic hospitals, the administration of preoperative CRT for oesophageal cancer patients was 200% of that in non-academic hospitals between 2001 and 2008. Since 2008, a rapid increase in the utilisation of preoperative CRT for oesophageal cancer was observed, that was similar in academic and non- academic hospitals (Supplementary Figure 1A, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1009638). The administration of preoperative CRT and CT in OGJ patients, and preoperative CT in gastric cancer patients in academic hospitals preceded non- academic hospitals by one year (Supplementary Figure 1C, D and F to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1009638). No consistent difference between the administration of preoperative CT for oesophageal cancer and postoperative CRT for gastric cancer was observed between academic and non-academic hospitals (Supplementary Figure 1B and E to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1009638). Additional multivariable logistic regression regarding 2000–2007 (results not shown) revealed that multimodality treatment receipt was significantly lower in non-academic than in academic hospitals for oesophageal cancer (HR = 0.73; p = 0.003). There was also a lower use of multimodality treatment in non-academic hospitals for OGJ and gastric cancer patients but this difference was not statistically significant (HR = 0.87; p = 0.473 and HR = 0.72; p = 0.063, respectively). There was no difference between academic and non-academic hospitals in multimodality treatment receipt during 2008–2012, for none of the three cancer sites.
Discussion
This nationwide population-based cohort-study in the Netherlands shows significantly increased utilisation of multimodality treatment for non-metastasised and potentially curatively resected oesophageal, OGJ, and gastric cancer over the past decade, 2000–2012. This increase occurred particularly in oesophageal and OGJ cancer, and was less pronounced in gastric cancer. This study also shows that multimodality treatment utilisation was significantly associated to lower age, higher clinical T and N classification, several CCC-regions and more recent time period. Additional analyses showed that the adoption of most types of multimodality treatment in academic hospitals preceded non-academic hospitals by a year. Also, in earlier years, before 2008, patients treated in academic hospitals were more likely to receive multimodality treatment.
Multimodality treatment trends
From 2000 until 2012 only up to half of the oesophagogastric cancer patients received multimodality treatment, indicating that the administration of multimodality treatment in unselected patients in daily clinical practice remains challenging [Citation8,Citation9, Citation12–14]. Nevertheless, when considering only the most recent years, we observed a large utilisation of multimodality treatment in oesophageal and OGJ cancer, and a moderate utilisation in gastric cancer. The higher multimodality treatment receipt rates in oesophageal and OGJ cancer than gastric cancer are in accordance with an US study [Citation8]. The rate of multimodality treatment for oesophageal and OGJ cancer is however, higher than previously described by others [Citation8,Citation9], which could be explained by the difference in studied time periods in combination with the more recent practice changing publications. For gastric cancer, similar multimodality receipt rates have been described in the US and Canada [Citation12, Citation13].
In oesophageal cancer, the increased use of preoperative CRT preceded the presentation of the (preliminary) results of the CROSS trial [Citation5], and the incorporation in the Dutch guideline. These findings are partially explained by patient inclusion from 2004 to 2008 in the Dutch CROSS trial by seven academic and three non-academic hospitals [Citation5], and several earlier indications that preoperative CRT was beneficial [Citation15,Citation16]. Furthermore, the implementation of preoperative CRT was extensive and fast, which could probably be explained by the usage of embedded logistics within the framework of the Dutch CROSS trial as radiotherapy institutes in the Netherlands are mostly independent from hospitals and patients need to be referred. Even though the implementation of preoperative CRT was early and possibly premature in the Netherlands, it seems that the extensive administration of preoperative CRT was even earlier in Sweden [Citation17].
In patients with OGJ cancer, overall preoperative CRT and preoperative CT were equally administered, likely in response to the MAGIC [Citation6] and the CROSS trial [Citation5]. Our observations reflect the option to treat OGJ cancer patients according either the oesophageal or gastric cancer guideline, which was also observed in the US [Citation8]. However, in contrast to other European countries, such as Denmark, Germany, England, Italy and France where standard treatment is perioperative CT, we observed a trend of more preoperative CRT in recent years. This could be explained by the more recent publication of the CROSS trial and national oesophageal guideline, but also the administration of this treatment within the Dutch CROSS trial accompanied by the embedded logistics.
In gastric cancer, after the publication of the INT0116 trial, only a slight increase in administration of postoperative CRT was observed. No further increase was observed after the start of the Dutch CRITICS trial in 2007, which investigates the additional advantage of postoperative CRT when combined with preoperative CT versus perioperative CT for resectable gastric cancer, and which is still actively recruiting (ClinicalTrials.gov number NCT00407186) [Citation18]. In comparison, an American cohort-study observed a 12% increase of the utilisation of postoperative CRT between 1999 and 2000, to an absolute 40% of treated patients thereafter in response to the INT0116 trial [Citation12,Citation13]. Similar trends were observed in a Canadian population-based study [Citation14]. Oppositely, in the Netherlands the utilisation of preoperative CT increased [Citation12]. This followed the results of the MAGIC trial [Citation6], preceding the national guideline. As mentioned before, the current Dutch guideline for gastric cancer recommends perioperative CT, and does not recommends postoperative CRT except for patients who did not receive preoperative CT and had an R1 resection [Citation19,Citation20]. Therefore, the more frequent choice in recent years for preoperative CT and its rapid increase might be explained by adherence of clinicians to the national guidelines, rather than responding to landmark publications.
Predictors of multimodality treatment and influence of hospital type
In literature, several patient and tumour characteristics in unselected oesophagogastric cancer patients have been consistently associated with a more frequent receipt of multimodality treatment, such as lower age, male gender, less comorbidity, higher income and higher tumour stage [Citation12]. In accordance, in this study, the receipt of multimodality treatment was associated with patient and tumour characteristics.
Besides patient and tumour characteristics, several other factors may also influence the receipt of multimodality treatment. The shift from surgery alone to multimodality treatment requires the rearrangement of cancer care, and synchronisation of logistics between disciplines and institutions. We hypothesised that this process will be more efficient for academic hospitals with more extensive resources. However, conflicting evidence on the association of hospital type and receipt of multimodality treatment has been described by others, indicating that hospital type as sole predictor of optimal cancer care is not sufficient [Citation21–23]. In this study, we found no independent influence of hospital type on the utilisation of multimodality treatment regarding the entire aggregated study period. Additional analyses showed however, that the implementation of several multimodality treatment regimens was earlier and faster in academic hospitals. Dividing the time period in the multivariable logistic regression analysis resulted in different effects of hospital type on multimodality treatment receipt, reflecting the higher multimodality treatment receipt in academic hospitals in earlier years independently of other factors. Therefore, the difference between academic and non-academic hospitals was especially pronounced before the implementation of the national guidelines. Thereafter, this difference was no longer present.
Limitations
This study has limitations inherent to large database studies. Importantly, co-morbidity was not scored in the used database, which is unfortunate because this influences treatment choices [Citation12], and could therefore introduce bias. Another limitation is the exclusion of patients who started with neo-adjuvant treatment, but did not proceed to potentially curative surgery for any reason whatsoever, because this would be more representative of daily clinical practice.
In conclusion, the utilisation of multimodality treatment for oesophagogastric cancer has increased in the Netherlands during the past decade. In oesophageal and OGJ cancer patients, preoperative treatment has become standard practice. For gastric cancer, however, 50% of patients were not treated with multimodality treatment. It remains debatable whether this is related to low compliance with the national guidelines, or to the frailty of the unselected patient population. Multimodality treatment receipt is especially dependent on patient and tumour characteristics and year of diagnosis. Multimodality treatment trends seem to be related to the publication of landmark studies, participation in nationally running clinical trials, and hospital type, preceding national guidelines especially in academic hospitals. Therefore, a more extensive and rapid distribution of practice changing study results could be valuable.
Supplementary material available online
Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1009638
ionc_a_1009638_sm1415.pdf
Download PDF (318.7 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Netherlands Cancer Registry. Available from: http://www.cijfersoverkanker.nl. [cited 2014 Dec 19].
- Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49.
- Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002; 347:1662–9.
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5– a population-based study. Lancet Oncol 2014;15:23–34.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20.
- Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG- directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327–33.
- Cronin-Fenton DP, Mooney MM, Clegg LX, Harlan LC. Treatment and survival in a population-based sample of patients diagnosed with gastroesophageal adenocarcinoma. World J Gastroenterol 2008;14:3165–73.
- Schonnemann KR, Mortensen MB, Bjerregaard JK, Fristrup C, Pfeiffer P. Characteristics, therapy and outcome in an unselected and prospectively registered cohort of patients with gastro-oesophageal cancer. Acta Oncol 2014; 53:385–91.
- Schouten LJ, Jager JJ, van den Brandt PA. Quality of cancer registry data: A comparison of data provided by clinicians with those of registration personnel. Br J Cancer 1993; 68:974–7.
- Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual, 7th ed. New York: Springer; 2010.
- Sherman KL, Merkow RP, Bilimoria KY, Wang CE, Mulcahy MF, Benson AB, et al. Treatment trends and predictors of adjuvant and neoadjuvant therapy for gastric adenocarcinoma in the United States. Ann Surg Oncol 2013; 20:362–70.
- Coburn NG, Guller U, Baxter NN, Kiss A, Ringash J, Swallow CJ, et al. Adjuvant therapy for resected gastric cancer– rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys 2008; 70:1073–80.
- Coburn NG, Lourenco LG, Rossi SE, Gunraj N, Mahar AL, Helyer LK, et al. Management of gastric cancer in Ontario. J Surg Oncol 2010;102:54–63.
- Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
- Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335:462–7.
- Rouvelas I, Zeng W, Lindblad M, Viklund P, Ye W, Lagergren J. Survival after neoadjuvant therapy compared with surgery alone for resectable esophageal cancer in a population-based study. World J Surg 2006;30:2182–90; discussion 91–2.
- Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329.
- Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430–6.
- Stiekema J, Trip AK, Jansen EP, Boot H, Cats A, Ponz OB, et al. The prognostic significance of an r1 resection in gastric cancer patients treated with adjuvant chemoradiotherapy. Ann Surg Oncol 2014;21:1107–14.
- Dikken JL, Dassen AE, Lemmens VE, Putter H, Krijnen P, van der Geest L, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer 2012;48:1004–13.
- Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–37.
- Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349: 2117–27.
