To the Editor,
Gastric or gastroesophageal junction (GEJ) adenocarcinoma represents a major health issue, being the third cause of death by cancer in the world. In industrialized countries, the incidence of GEJ cancers continues to rise, while gastric cancers have declined over the past few decades.
The human epidermal growth factor 2 (HER2) gene is a well described proto-oncogene. HER2 gene amplification and protein overexpression have been mostly studied in breast cancer, where HER2 overexpression was reported in 15–20% of cases.
In breast cancer, HER2 overexpression is associated with a higher risk of brain metastases and significantly worse survival [Citation1].
The prognostic value of HER2 overexpression in gastric and GEJ adenocarcinomas remains controversial. A recently published systematic review was inconclusive in patients after curative surgery [Citation2]. The standard of care in advanced gastric carcinoma patients has changed recently, with the results of the ToGa phase III trial adding trastuzumab to chemotherapy in patients with HER2 overexpression [Citation3]. The study indicated that this subgroup of patients benefit from trastuzumab with a three-month improvement of overall survival (OS).
The aim of this study was to seek if the correlation between HER2 overexpression and brain metastases exists in gastric or GEJ adenocarcinomas, where brain metastases are uncommon.
Material and methods
Patients
We collected retrospectively clinical and histological data on patients treated between 2007 and 2011 for metastatic gastric, GEJ or lower third esophageal adenocarcinoma from hospital medical charts of our institution. Data included location of the primary tumor, TNM staging, histological subtype, adjuvant chemotherapy and/or radiotherapy, time to recurrence, site of metastases, treatment of metastatic disease, occurrence of cerebral localization, chemotherapy regimens, response to treatment, time to tumor progression and OS.
Immunohistochemistry
IHC were assayed on 4 μm sections of formalin-fixed paraffin-embedded tumor tissues using primary polyclonal antibody A085 (Dako) using standard methodology. Cases were considered positive if scored 3+, negative if scored 0 to 1+, FISH was performed in cases scored 2 + by IHC [Citation4].
Fluorescent in situ hybridization
Interphase FISH analysis was performed on 4 μm sections of formalin-fixed paraffin-embedded tumor tissues using the PathVysion HER-2 DNA Probe Kit (Abbott Molecular, Rungis, France). Cases were considered positive if the ratio HER2 (17q11.2-12)/ CEP17 (17p11.1-q11.1 Alpha Satellite DNA) was superior to 2.2 [Citation4].
Statistical methodology
We compared the frequency of brain metastases from two groups, one HER2 + and the other HER2-, with Fisher's exact test. Survival analysis was performed by the Kaplan-Meier method for OS and progression-free survival (PFS).
Results
Between 2007 and 2011, 74 patients were treated for metastatic gastric or GEJ adenocarcinoma at our institution, and HER2 status could be analyzed in 63. Eleven patients were excluded for missing histopathological data.
Demographic and clinical characteristics of patients are summarized in .
Table I. Clinicopathological parameters and HER2 status in 63 gastric adenocarcinoma.
Eleven (18%) specimens were HER2-positive (HER2+). Status were obtained by immunohistochemistry in 58 patients, FISH in five. The median age was 62 years (range 22–81), there were 52 men (83%) and 11 women (18%). Thirty-six patients (57%) had a gastric tumor, 27 (43%) tumor of the GEJ. After a median follow-up of 9.4 months, 52 patients showed progression, and 38 patients died. All patients had chemotherapy either initially or at the time of metastatic evolution. Median PFS was 5.3 months and median OS was 15.9 months. Thirty-eight patients (60%) had liver metastases, 32 (51%) lymph node metastases, 20 (32%) peritoneal carcinomatosis, 12 (19%) lung metastases and three (5%) brain metastases.
Two patients HER2+ did not receive trastuzumab because their treatment was performed before the result of the TOGA study. In the 11 patients HER2+, 3 (27%) had brain metastases, versus 0 (0%) in the 52 HER2- patients (Fisher's exact test p = 0.004). Brain metastases were all discovered after appearance of neurological symptoms (motor deficit, headache, and stroke). One occurred during treatment with trastuzumab. The control of brain metastases was obtained in all three patients with a multimodal treatment (surgery for one patient, radiotherapy for three patients and chemotherapy associated with trastuzumab for three patients). One of the patients was polymetastatic, and the other two had either lymph node or liver metastases. The response to treatment of brain lesions was heterogeneous with one partial response, and two stable disease.
Moreover, peritoneal carcinomatosis was diagnosed in 20 of the 52 (39%) HER2- patients versus 0 of 11 (0%) HER2+ patients (p = 0.013). There was no association between HER2 status and other sites of metastases, and no association between brain or peritoneal carcinomatosis and other factors (gender, age, T and N stage).
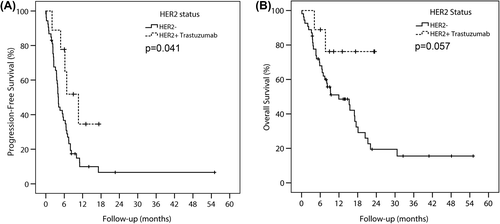
We studied PFS and OS as an exploratory analysis, due to small numbers. PFS was significantly better among HER2+ patients treated with trastuzumab than in patients with HER2-: median 9.4 months against 5.0 months (p = 0.041) (). The respective median OS was not reached and 10.9 months, but the difference was not statistically significant (p = 0.057) ().
Discussion
The prognostic value of HER2 gene amplification and protein overexpression in gastric adenocarcinoma is not as clear as in breast cancer. The largest study to date with 900 cases found that HER2 expression in gastric cancer was not correlated to prognosis. In this study less than 10% of all gastric cancer expressed HER2 [Citation5]. In a review of the 35 studies reporting the impact of HER2 overexpression on survival, 44% of patients had TNM stage I/II, and 56% had TNM stage III/IV disease. Twenty studies (57%) reported no difference in OS, two studies (6%) reported significantly longer OS in patients with HER2 overexpression and 13 studies (37%) reported significantly poorer OS in patients with HER2 overexpression [Citation2]. Overall it appears difficult to determine to the prognostic value of HER2 overexpression.
In breast cancer, HER2+ patients had an increased risk of brain metastases [Citation6,Citation7], and the development of central nervous system (CNS) metastases in breast cancer is associated with poor prognosis [Citation6]. However, to date similar data on HER2+ gastric adenocarcinoma and brain metastases are still lacking. In this retrospective single-center series, we tried to offer some preliminary data. We showed increased incidence of brain metastases in patients with HER2+ gastric or GEJ adenocarcinomas. Three HER2+ patients had cerebral metastases, as compared with HER2- (p = 0.004). Another previously unreported finding of this study is the less frequent peritoneal carcinomatosis in the HER2+ group. This could be related to the fact that HER2 overexpression is less frequent for diffuse type gastric cancer [Citation2]. Diffuse-type gastric cancers have been associated with more frequent peritoneal carcinomatosis. These differences in pattern of metastases are probably under the dependence of different biological behavioral, which may gain importance in the next years for decision-making.
Limits of our study are clearly its monocentric nature and the small number of patients. Our results should be replicated by other group. However, in the current study, all histological specimens were assessed for HER2 status as recommended by Rüschoff et al., with initial IHC testing and FISH/silver in situ hybridization to re-evaluate samples with an IHC score of + 2 [Citation4].
In conclusion, in this single-center retrospective study, we showed an increased incidence of brain metastases in patients with HER2 + gastric or GEJ adenocarcinomas, and a decreased incidence of peritoneal carcinomatosis. The prognosis of patients HER2+ treated with trastuzumab seems better than that of patients HER2+ . These results should be confirmed in a multicenter study. The possible consequence in this population would be the necessity of a systematic search for brain metastases.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Altaha R, Crowell E, Hobbs G, Higa G, Abraham J. Increased risk of brain metastases in patients with HER-2/neu-positive breast carcinoma. Cancer 2005;103:442–3.
- Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes – a systematic review. Int J Cancer 2012;130: 2845–56.
- Bang YJ, Van CE, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
- Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: A practical approach. Mod Pathol 2012;25:637–50.
- Grabsch H, Sivakumar S, Gray S, Gabbert HE, Muller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value – conclusions from 924 cases of two independent series. Cell Oncol 2010;32:57–65.
- Minisini AM, Moroso S, Gerratana L, Giangreco M, Iacono D, Poletto E, et al. Risk factors and survival outcomes in patients with brain metastases from breast cancer. Clin Exp Metastasis 2013;38:951–6.
- Ahn HK, Park YH, Lee SJ, Park S, Maeng CH, Park W, et al. Clinical implication of Time To Brain Metastasis (TTBM) according to breast cancer subtypes. Springerplus 2013;2:136.