Abstract
Background. Neoadjuvant chemotherapy has proven valuable in several tumors, but it has not been elucidated in colon cancer. The present phase II trial addressed the issue in high-risk patients selected by computed tomography (CT) scan.
Material and methods. Patients with resectable colon cancer fulfilling the following criteria were offered inclusion; Histopathological verification of adenocarcinoma, T3 tumor on CT scan with extramural tumor invasion > 5 mm or T4 tumor, age ≥ 18 years, PS ≤ 2, adequate hematology, and informed consent. Patients with KRAS, BRAF or PIK3CA mutation or unknown mutational status received three cycles of capecitabine 2000 mg/m2 days 1-14 q3w and oxaliplatin 130 mg iv day 1 q3w. Wild-type patients received the same chemotherapy supplemented with panitumumab 9 mg/kg iv q3w. After the operation, patients fulfilling the international criteria for adjuvant chemotherapy, i.e. high-risk stage II and III patients, received five cycles of the same chemotherapy without panitumumab. Patients not fulfilling the criteria were offered follow-up only. The primary endpoint was the fraction of patients not fulfilling the criteria for adjuvant chemotherapy (converted patients). Secondary endpoints were recurrence rate, disease-free survival (DFS), and toxicity.
Results. The study included 77 patients. The conversion rate was 42% in the wild-type group compared to 51% in patients with a mutation. The cumulative recurrence rate in converted versus unconverted patients was 6% versus 32% (p = 0.005) translating into a three-year DFS of 94% versus 63% (p = 0.005).
Conclusion. Neoadjuvant chemotherapy in colon cancer is feasible and the results suggest that a major part of the patients can be spared adjuvant chemotherapy. Validation in a randomized trial is warranted.
Locally advanced colon cancer represents a major therapeutic challenge. Despite recent development in surgical technique [Citation1] and non-surgical treatment [Citation2] a considerable part of the patients will die from the disease. A Cochrane review [Citation3] showed that adjuvant chemotherapy improves disease-free survival (DFS) in stage II, but it had no effect on overall survival (OS). Similar results appear from the US Medicare database [Citation4]. There was no difference in five-year OS in the study of 24,847 stage II patients with a fraction of 20% receiving adjuvant chemotherapy. The lack of effect applied not only to patients with favorable histopathological characteristics, but also to patients with poor prognostic features according to the ASCO classification [Citation5].
There is a moderate effect of adjuvant chemotherapy in patients with stage III disease. The difference in DFS between surgery alone and adjuvant 5-FU with leucovorin is around 10% and further improvement can be obtained with FOLFOX [Citation6]. Combination chemotherapy is, however, dubious in elderly patients [Citation7] and the recent NSABP trial [Citation8] did not confirm the better effect. Furthermore, the long-term toxicity of combination chemotherapy should not be neglected [Citation9].
Targeted adjuvant treatment has been disappointing. A randomized NCCTG trial [Citation10] compared combination chemotherapy with and without cetuximab. The results did not demonstrate any benefit of cetuximab, neither in patients with KRAS mutated tumors nor in KRAS wild-type patients. The AVANT trial [Citation11] investigated the effect of bevacizumab added to oxaliplatin-based chemotherapy. There was no improvement, neither in DFS nor in OS. The systemic adjuvant treatment has probably reached a plateau calling for new treatment approaches.
Neoadjuvant treatment has demonstrated beneficial effect in several malignant tumors and now forms part of the standard treatment in e.g. breast, rectal, esophageal, and gastric cancer, but it has not been systematically investigated in colon cancer. One major concern has been the risk of progression during chemotherapy with bowel obstruction needing emergency surgery. It is well known that such a condition implies a poor prognosis. This risk, however, is small with modern combination chemotherapy and very few patients progress during the first three cycles. On the other hand, there has been a concern as to overtreatment due to shortness of validated methods for preoperative selection of high-risk patients.
Recent technical development in computer tomography (CT) has changed the situation. A study including 312 patients undergoing resection of colon cancer indicated that CT scanning could classify the patients into two prognostic groups with a three-year recurrence-free survival of 71% and 43%, respectively [Citation12]. A further study with multidetector CT scanning has confirmed that CT is an accurate method of identifying patients with a poor prognosis and consequently, candidates for neoadjuvant treatment [Citation13]. Similar results have been reported in a meta-analysis concluding that CT scanning accurately separates tumors confined to the bowel wall from those with extramural tumor invasion (ETI) [Citation14], and an ongoing trial has confirmed that CT scanning can identify patients with high-risk T3 and T4 tumors with minimal overstaging [Citation15]. The feasibility of the trial including 150 patients showed that 86% had characteristics indicating a poor prognosis [Citation16]. Taken together, there is solid evidence that the application of multidetector CT scanning is a suitable method of selecting patients for neoadjuvant chemotherapy.
The aim of the present study was to investigate whether neoadjuvant chemotherapy can convert high-risk patients (needing adjuvant chemotherapy) to a low-risk status (not fulfilling the criteria for adjuvant chemotherapy). Furthermore, we wanted to explore the effect of targeted treatment based on relevant markers.
Material and methods
Study design
The study was an open, multicenter phase II trial in patients with locally advanced, but resectable colon cancer. The patients were divided into two subgroups according to mutational status. Patients with KRAS, BRAF or PIK3CA mutations were offered three cycles of combination chemotherapy. The same applied to patients with unknown mutational status at the time of inclusion. Patients with documented wild-type in all three loci were offered the same chemotherapy supplemented with panitumumab. The treatment was scheduled to start within two weeks after inclusion and delay because of mutation analysis was not accepted.
Patients
The inclusion criteria were; histopathological verification of adenocarcinoma, CT scan showing a T3 tumor with ETI > 5 mm or a T4 tumor, no metastases on chest and abdominal CT, PS ≤ 2, age ≥ 18 years, planned analyses of KRAS, BRAF, and PIK3CA mutations. Patients with unknown mutational status could be included for treatment with chemotherapy alone without targeted treatment. ANC ≥ 1.5 × 109/l and thrombocytes ≥ 100 × 109/l. Bilirubin ≤ 3 × upper normal value, and ALAT ≤ 5 × upper normal value. Negative pregnancy test and informed consent. Exclusion criteria were; serious infections, peripheral neuropathy > NCE grade 1, significant cardiac disease with myocardial infarction, unstable angina, uncontrolled arrhythmia ≤ 1 year before inclusion. Other malignant diseases within five years before inclusion except non-malignant melanoma skin cancer and carcinoma in situ cervicis uteri.
Efficacy and safety assessment
The primary endpoint was conversion rate expressed as the fraction of patients not fulfilling the criteria for adjuvant chemotherapy, i.e. stage II without the following characteristics; poorly differentiated tumor, pT4, vascular lymphatic or perineural invasion, < 12 lymph nodes in the surgical specimen, or stage III [Citation17]. Secondary endpoints were recurrence rate, DFS, safety and feasibility of the treatment.
Adverse events were scored according to the CTCAE criteria version 3. The treatment with cytostatics was postponed one week in case of ≥ grade 2 bone marrow toxicity and the dose reduced to 50% in case of febrile leucopenia. Oxaliplatin was reduced to 75% in case of parasthesia lasting > 7 days but < 14 days and discontinued if parasthesia was present through the whole interval between two cycles. Administration of panitumumab was postponed in case of grade 3 skin toxicity ascribed to panitumumab and continued at a 50% dose after complete recovery. The treatment was discontinued in case of grade 4 toxicity.
CT scans
CT scans were performed using 64-channel multidetector equipment (Brilliance 64, Philips, Eindhoven, the Netherlands) with a section thickness of 3 mm. All CT examinations underwent central review for verification of tumor extension. A CT scan was performed before start of treatment, before the operation, and six, 12, and 24 months after end of treatment.
Mutation analysis
The KRAS and BRAF mutation analyses were performed with a method described in detail previously [Citation18]. The method detects six KRAS mutations in codon 12 (Gly12Ala, Gly12Arg, Gly12Asp, Gly12Cys, Gly12Ser, and Gly12Val) and one mutation in codon 13 (Gly13Asp) in addition to one BRAF mutation (V600E). Mutations in the PIK3CA gene were detected by means of the PI3K Mutation Test Kit, catalogue no. PK-02 (Qiagen). The kit was used according to the manufacturer's guidelines and detected four mutations (H1047R, E545K, E542K, and E545D).
Treatment
The neoadjuvant treatment consisted of three cycles of XELOX (capecitabine 2000 mg/m2 orally days 1-14 q3w, oxaliplatin 130 mg/m2 iv day 1 q3w). The additional neoadjuvant panitumumab for wild-type patients was given as 9 mg/kg iv q3w for three cycles. The adjuvant treatment included five cycles of chemotherapy identical to that of the neoadjuvant treatment except for panitumumab, which was omitted in the adjuvant setting.
The resection was performed three weeks after the last cycle of chemotherapy according to standard national criteria. Both laparoscopic and open surgery was accepted.
The biopsies and surgically removed specimens were examined according to standard pathology criteria and classified according to the pTNM system. One of the authors (JL) performed a central pathology review of histomorphological risk factors, including extramural venous and neural invasion and tumor grade.
After the operation patients fulfilling the criteria for adjuvant treatment received five cycles of chemotherapy. Patients not fulfilling the criteria for adjuvant chemotherapy were offered follow-up only. The follow-up was every three months the first year. In the second and third year follow-up was scheduled every six months.
Statistics
The calculation of sample size was based on Simon's two-stage minimax design [Citation19]. It was anticipated that at least 75% of the patients were in the high-risk group, and the study was interesting for a phase III trial, if this group was reduced to 50% after neoadjuvant treatment. Based on a significance level of 5% and a power of 90% the first part of the trial was to include 16 patients. If ≤ 4 were in the low-risk group, the study would be terminated. Otherwise the study would continue until inclusion of 33 patients. It was decided to work up the two treatment groups separately and consequently, the study would include 66 evaluable patients. To compensate for dropouts and uncertainty on the frequency of different mutations the study aimed at 76 patients.
DFS was calculated from the day of inclusion to the day of recurrence or death from any cause. Survival was calculated as Kaplan-Meier plots and compared by the log-rank test. Categorial variables were compared using Fisher's exact test.
Results
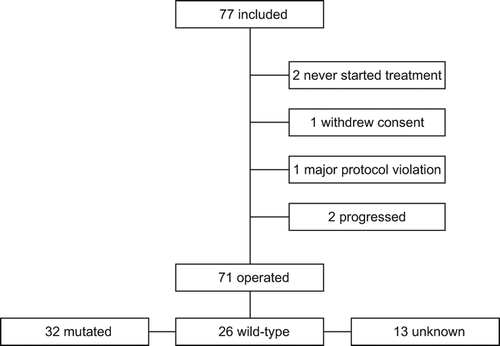
The study included 77 patients between August 2010 and August 2013. The consort diagram () shows that two patients never started treatment; one because of ileus and one due to renal disease. One patient withdrew his consent, and major protocol violation was the reason for exclusion of one patient. Two patients progressed during treatment; one had distant metastases on the preoperative CT scan and the other one presented with a non-resectable tumor, leaving 71 patients for operation.
shows the patient characteristics. It appears that 85% had an advanced T3 tumor with ETI > 5 mm and 15% a T4 tumor, and all but one were classified as N+ by the primary CT scan. All patients were in good general condition with PS 0 or 1. The table also shows that 63% of the patients harbored a tumor with a mutation.
Table I. Patient characteristics.
presents the primary endpoint in the two groups. There is no major difference, but less than half of the wild-type patients (42%) were converted to a low-risk status compared to 51% in the chemotherapy only group for a total conversion rate of 48%.
Table II. Conversion according to mutational status (N = 71).
The comparison of clinical T-category based on the primary CT scan and pT-category is shown in . It clearly appears that most tumors did not change T-category, but 34% converted to a lower T-category and 11% were classified a higher one at pathological examination of the surgical specimens. It is also remarkable that complete regression was found in three cases, none of them with lymph node metastases, and they thus had complete pathological remission (CPR). The table also gives the N-category. Lymph node metastases were found in 34% of the patients, most of them in the N1-category. Thus, 66% were converted to N0-status compared to the primary CT scan. In the converted patients 68% were still stage II after neoadjuvant chemotherapy.
Table III. Comparison between primary CT scan and pathology (N = 71).
Generally, the preoperative treatment was well tolerated with 83% of the patients receiving all three cycles of chemotherapy and 90% receiving at least two cycles. The bone marrow toxicity was mild with no episodes of febrile leucopenia. The same applied to neurotoxicity with only one patient presenting with grade 3 parasthesia. The severe toxicity is shown in . Not surprisingly, a considerable part of the patients in the panitumumab group experienced serious skin toxicity resulting in dose reduction in half of the patients. There were no signs of increased local symptoms and no patient had the operation postponed because of chemotherapy. The major surgical complications were; intraabdominal abscess 0%, ileus 4%, dehiscence 3%, anastomotic leakage 7%. There was no obvious difference between the treatment groups. The median postoperative stay in hospital was six days and there was no operative (30 days) mortality. Seventy-five percent of the patients completed the planned adjuvant chemotherapy.
Table IV. Toxicity ≥ grade 3 and dose reduction.
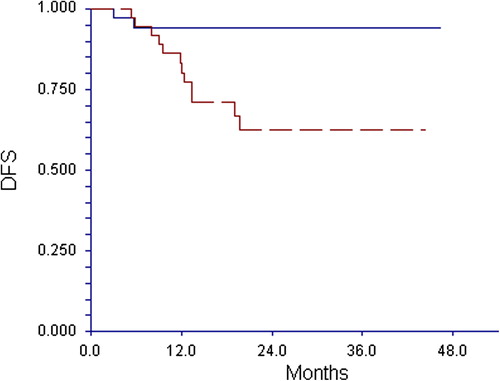
The recurrence rate differed significantly according to conversion. Only two patients (6%) recurred in the converted group compared to 32% in the unconverted group (p = 0.005). The difference was reflected in DFS (). With a median observation time of 26.4 months the three-year DFS was 94% in the converted group and 63% in the unconverted group (p = 0.005).
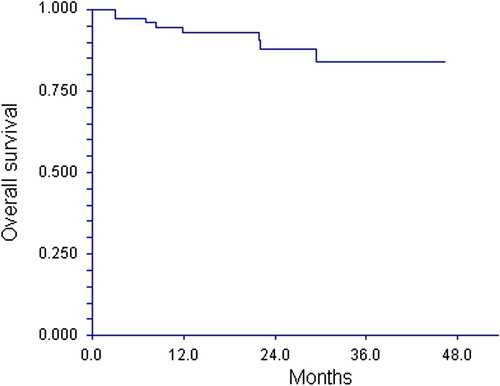
The OS by intention to treat appears from . The Kaplan-Meier plot indicates a two- and three-year cumulative survival of 88% and 84%, respectively.
Discussion
Neoadjuvant chemotherapy is a well established concept in clinical oncology. It has several theoretical advantages compared to the adjuvant setting. First, the oxygen supply may be better in the preoperative situation compared to postoperative conditions with fibrosis and poor blood supply to the area. Second, in colon cancer preoperative treatment may also reduce the risk of tumor cells spreading due to the surgical trauma. Finally, it may also eliminate lymph node micrometastases and circulating tumor cells.
The results presented here show that neoadjuvant chemotherapy in colon cancer is feasible. The side effects seem to be in agreement with the pattern seen in the treatment of metastatic disease using the same drugs. Two patients were classified with progression. One presented a lung metastasis on the preoperative CT scan verified by a PET scan. The other patient turned out to have a non-resectable tumor. It is difficult to judge whether it was in fact non-resectable already at the start of treatment. Anyhow, progression during treatment represents only a minor problem. The rate and type of surgical complications are in agreement with the current literature dealing with patients not receiving neoadjuvant chemotherapy. The same applies to the stay in hospital.
The risk of overtreatment cannot be neglected, as there was no histopathological verification of tumor stage before start of treatment. However, our recent study demonstrated that T3 patients with ETI > 5 mm or a T4 tumor fulfil the criteria for adjuvant chemotherapy, when operated without preoperative treatment [Citation20] in agreement with the current literature. Consequently, it seems reasonable to anticipate that at least 75% of the patients were in the high-risk group before start of treatment. The conversion rate found in our study relies partially on an elimination of lymph node metastases. In patients with locally advanced disease a rate of at least 50% is expected compared to 33% in our study. The high conversion rate in comparison with the primary CT scan should be interpreted with caution, since the accuracy of CT as to lymph node metastases is low. Regression of the primary tumor is also likely to play a role as illustrated by a conversion of 34% of the tumors to a lower T-category.
The study was not designed to show a difference between the treatment groups, but there was no signal as to any benefit of adding panitumumab, neither with respect to effect nor toxicity. In fact, it did not meet the primary endpoint as opposed to chemotherapy alone. Panitumumab may be a bad companion to oxaliplatin as suggested from the treatment of metastatic colorectal cancer [Citation21]. This is clearly underlined by data from the New EPOC study indicating a worse outcome in the cetuximab arm [Citation22]; an issue not raised when the present trial was planned.
The mutation pattern found here to some extent is different from that in the literature, especially with respect to the frequency of BRAF mutations. This may happen just by chance, but it may also be at least partially explained by the high fraction of right-sided tumors, which has been shown to imply a high risk of BRAF mutation [Citation23]. The high frequency of BRAF mutations is outbalanced by a proportionally low frequency of KRAS mutations. The explanation is not likely to be the method used. Its quality was proven by participation in the European Society of Pathology external quality assurance program. The application of PIK3CA mutation for selection of treatment with panitumumab may be criticized, but at the start of the study the literature on the issue was not too clear. A significant paper [Citation24] claimed that PIK3CA status was important to the effect of EGFR antibodies.
There is no trial similar to the one presented here. A number of case reports and small cohorts appear in the literature [Citation25], but no finalized, systematic trials have been presented. Our trial is also different from the ongoing phase III trial in two essential aspects. First, the chemotherapy was given for nine weeks in our trial compared to six weeks in the FOxTROT study [Citation16], and second, our study took consequence of the response to neoadjuvant chemotherapy by omitting adjuvant treatment in the converted group.
The recurrence rate in the converted group proved very low despite no further treatment and translated into a high DFS. This is probably not surprising. Neoadjuvant chemotherapy seems to select a group of patients with a high response rate and a good prognosis, but a DFS of 94% is remarkable for a group of patients with 68% being stage II. Treatment selection based on response to neoadjuvant chemotherapy is an ongoing discussion in breast cancer, but it has not been started in colon cancer.
The limitations of the study should not be forgotten. First of all, it is a phase II trial and consequently it does not allow any conclusion as to the importance of neoadjuvant chemotherapy in the general patient population. Also, the observation time is too short to allow conclusion on the long-term recurrence rate. It cannot be definitively excluded that neoadjuvant chemotherapy only delays the recurrence, but it seems less likely, since the pattern in the unconverted group is in agreement with that of patients not receiving neoadjuvant chemotherapy.
Conclusion
In conclusion, the results presented here show that neoadjuvant chemotherapy in colon cancer is feasible. The data suggest high efficacy of the treatment but call for validation in a randomized trial, especially the issue of no further treatment after operation, aiming at “less treatment, better results”.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation – technical notes and outcome. Colorectal Dis 2009;11:354–64.
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev 2008;3:CD005390.
- O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381–8.
- Benson III Al B, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408–19.
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the Mosaic trial. J Clin Oncol 2009;27:3109–16.
- Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: Subgroup analyses of the multicenter international study of oxaliplatin, fluorouracil, and leucovorin in the adjuvant treatment of colon cancer trial. J Clin Oncol 2012;30:3353–60.
- Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29:3768–74.
- Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707.
- Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA 2012;307:1383–93.
- Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Lopa SH, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359–64.
- Smith NJ, Bees N, Barbachano Y, Norman AR, Swift RI, Brown G. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: Implications for clinical trials. Br J Cancer 2007;96:1030–6.
- Dighe S, Blake H, Koh MD, Swift I, Arnaout A, Temple L, et al. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg 2010;97:1407–15.
- Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A’Hern R, et al. Diagnostic precision of CT in local staging of colon cancers: A meta-analysis. Clin Radiol 2010;65:708–19.
- Dighe S, Swift I, Magill L, Handley K, Gray R, Quirke P, et al. Accuracy of radiological staging in identifying high-risk colon cancer patients suitable for neoadjuvant chemotherapy: A multicentre experience. Colorectal Dis 2012;14:438–44.
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: The pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152–60.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479–516.
- Spindler KL, Pallisgaard N, Vogelius I, Jakobsen A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin Cancer Res 2012;18:1177–85.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10.
- Nørgaard A, Dam C, Jakobsen A, Pløen J, Lindebjerg J, Rafaelsen SR. Selection of colon cancer patients for neoadjuvant chemotherapy by preoperative CT scan. Scand J Gastroenterol 2012;49:202–8.
- Grothey A, Lenz HJ. Explaining the unexplainable: EGFR antibodies in colorectal cancer. J Clin Oncol 2012;30: 1735–7.
- Primrose JN, Falk S, Finch-Jones M, Valle JW, Sherlock D, Hornbuckle J, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol 2014;15:601–11.
- Chen D, Huang J-F, Liu K, Zhang L-Q, Yang Z, Chuai Z-R, et al. BRAFV600E mutation and its association with clinicopathological features of colorectal cancer: A systematic review and meta-analysis. PLOS One 2014; 9:e90607.
- Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009;69: 1851–7.
- Arredondo J, Pastor C, Baixauli J, Rodríguez J, González I, Vigil C, et al. Preliminary outcome of a treatment strategy based on perioperative chemotherapy and surgery in patients with locally advanced colon cancer. Colorectal Dis 2013;15:552–7.