Abstract
Background and purpose. Metabolism in tumor cells depends mainly on glycolysis and thus hyperglycemia has been shown to influence tumor properties in various tumor entities. In this retrospective study we set out to determine if hyperglycemic serum levels during radiation therapy impact patient survival and progression patterns in primary glioblastoma (GBM).
Material and methods. We retrospectively analyzed glucose serum levels, survival and progression patterns on magnetic resonance imaging (MRI) in 262 GBM patients receiving radiation therapy. Hyperglycemia was classified as mild (> 180 mg/dL) or excessive (≥ 300 mg/dL), and isolated (one hyperglycemic event) or persistent (≥ 3 hyperglycemic events). The multivariate Cox proportional hazards ratio was used to assess the influence of cofactors on survival.
Results. Persistent mild (HR = 2.23; p < 0.001) and excessive hyperglycemia (HR = 2.51; p < 0.001) were associated with a decrease in overall survival rates, even when considering the covariate corticosteroid therapy. Here metabolic imbalances did not affect the progression-free interval (p = 0.402), the occurrence of distant (p = 0.587) and multifocal progression (p = 0.445).
Conclusion. Our findings support the theory that hyperglycemia during radiation therapy in GBM patients is an unfavorable prognostic cofactor for survival and is detrimental to the survival rates independent of corticosteroid therapy. However, no significant effects of hyperglycemic metabolism on the progression-free interval and recurrence patterns were found.
Glioblastomas (GBMs) represent the most frequent primary malignant tumors of the central nervous system in adults [Citation1]. Although treatment options have improved during the last few years, the median overall survival (OS) after standard therapy with surgical resection followed by chemoradiotherapy remains poor, being approximately 14.6 months [Citation2].
Interestingly, individual survival is heterogeneous and long-term survival is possible [Citation3]. Few clinical prognostic factors, e.g. age, gender, performance status and tumor localization have been identified to be associated with survival [Citation4,Citation5].
Over the past few years researchers have therefore intensified their efforts to determine prognostic biomarkers in GBM. A valuable approach could be the regulation of a hyperglycemic metabolism during radiotherapy.
Several studies have demonstrated that hyperglycemia is associated with decreased survival rates in various tumor entities [Citation6–8]. In the 1930s Otto Wartburg first described that carcinoma cells gather their energy mainly through fermentation of sugar, even though sufficient energy is provided through respiration [Citation9,Citation10].
Following Warburg's hypothesis, a reduction in the available amount of glucose may induce apoptosis in tumor cells and lead to a reduction in tumor mass and tumor progression.
Jelluma et al. were able to underscore these findings by withdrawing glucose in GBM cell lines. They found that hypoglycemic metabolism leads to high apoptosis rates in GBM cells [Citation11].
Previously, hyperglycemia has been shown to influence recurrence behavior in other tumor sites [Citation12–14]. In GBM, death occurs very likely through local or distant tumor progression. It is unknown whether the correlation between hyperglycemia and death in GBM patients is due to an unfavorable recurrence behavior with multifocal or distant progression. Interestingly, the influence of hyperglycemic metabolism on recurrence patterns in GBM has not yet been investigated. Multiple cytoregulatory cofactors in patients with hyperglycemia might influence GBM cell properties that impact a relapse of disease. Several clinical studies show an association of hyperglycemia and decreased survival in glioma patients [Citation15,Citation16].
Although earlier findings provided new insights, the role of hyperglycemia in GBM patients has remained unclear.
This circumstance was our incentive to determine the effects of hyperglycemic serum levels during radiation therapy on patient survival and progression behavior in primary GBM.
Patients and methods
A total of 262 selected patients with histologically proven primary GBM were considered in this retrospective analysis. All were treated with radiotherapy between January 2008 and December 2011. Inclusion criterion was the availability of at least three glucose serum levels during radiation therapy.
In total 163 male (62.2%) and 99 female (37.8%) patients with a median age of 63.2 years (range 19.9–82.9 years) were studied.
Clinical, surgical and hospital course records including post-operative magnetic resonance imaging (MRI) were reviewed. The pretherapeutic Karnofsky performance status (KPS) was determined for all patients. All data were assessed per institutional guidelines in accordance with the declaration of Helsinki and the study was approved by the local ethics committee (Nr. S-056/2015). Neuropathological diagnosis was updated following the most recent WHO classification [Citation1].
Radiotherapy/Chemoradiotherapy
A median dose of 60.0 Gy (range 34.0–68.0 Gy) was administered in 2.0 Gy daily fractions (range 1.8–3.0 Gy per day). A total of 165 patients (63.0%) received combined temozolomide therapy, while radiation therapy alone was performed in 96 cases (36.6%). In one case the therapeutic regime could not be determined with certainty. All patients enrolled in the current study completed the radiotherapy protocol. Due to the heterogeneity of patients, several fractionation schemes were used during the period of investigation.
The target volume definition was based on the T1-weigthed contrast-enhanced MRI, including the primary tumor region and the T2-FLAIR hyperintense. Additionally, a 2–3 cm safety margin was added to cover potential microscopic tumor spread.
Hyperglycemic serum levels
Fasting glucose levels were not available for the entire patient collective. Laboratory tests, including serum glucose levels, were performed weekly, but the frequency was not standardized.
At least three valid in- and outpatient laboratory serum glucose evaluations were available for all patients during radiation therapy. Serum glucose levels were classified as mild hyperglycemia (180–299 mg/dL) [Citation15] and excessive hyperglycemia (≥ 300 mg/dL) [Citation17]. Furthermore, hyperglycemia was categorized regarding the probability of occurrence, in single event and persistent (≥ 3 events). Those patients who received dexamethason at the time of discharge were defined as: “with corticosteroid therapy at the time of dismissal”. Diabetes was defined as diabetes mellitus in advance of surgical resection and radiation therapy.
Data were generated by following all patients prospectively at our institution six weeks after radiotherapy and also by following a three-month interval until progress. MRI–based follow-up examinations until disease progression or death were performed.
Imaging
Preoperative MRI and CT-based treatment planning as well as post-operative follow-up MRI was available at an image archiving and communication system (PACS) based in the Department of Radiation Oncology/University Hospital Heidelberg, Germany. Tumor and relapse localizations were determined by an experienced radiological specialist (T.B.), as described previously [Citation3]. Only patients with sufficient follow-up MRI (n = 186) were included in the relapse pattern analysis.
Statistical analysis was carried out using SigmaPlot™ (Systat Software GmbH, Germany). The Kaplan-Meier survival analysis was performed for a progression-free and an OS analysis. A univariate and multivariate Cox proportional hazards ratio (HR) was used to assess the influence of cofactors on survival.
The correlation of patients’ characteristics in subtypes was determined by using the odds ratios and corresponding two-sided 95% confidence intervals. A p-value of < 0.05 was considered as statistically significant.
Results
In total 262 patients were included in this retrospective evaluation. A total of 185 patients (70.6%) had previously undergone surgical tumor resection. Gross total resection was completed in 82 cases (31.3%). In 73 cases (27.9%) GBM diagnosis was confirmed by biopsy only. The margin status of resection could not be determined in four patients (1.5%).
The O-6-methylguanine-DNA methyltransferase (MGMT) promoter status was assessable in 90 cases (34.4%), of which 39 (43.3%) were hypermethylated.
Patient hyperglycemia
In total 125 patients (47.7%) experienced hyperglycemic episodes during radiotherapy. In 30 cases (11.5%) an isolated mild hyperglycemic event (between 180 and 299 mg/dL) occurred, while 95 patients (36.3%) experienced persistent mild hyperglycemia (> 3 times: 180–299 mg/dL); 17 patients (6.5%) experienced isolated excessive hyperglycemia (≥ 300 mg/dL); and 45 patients (17.2%) experienced persistent excessive hyperglycemia (≥ 300 mg/dL). A total of 32 patients (12.2) had a history of diabetes mellitus. Diabetic patients were more likely to have persistent excessive hyperglycemia (OR: 5.27; CI 95% 2.44–11.37; p < 0.0001).
Survival analysis
The median progression-free survival (PFS) of the study group was 6.94 months (range 5.78–8.09 months) and the OS was 14.85 months (12.6–17.11 months). Simultaneous temozolomide therapy was significantly associated with increased PFS (p = 0.042). Isolated and persistent mild (isolated: p = 0.645; persistent: p = 0.517) and excessive (isolated: p = 0.088; persistent: p = 0.245) hyperglycemia did not show a significant effect on the PFS (, and ). There was no significant association of cofactors with the time to progression but concomitant temozolomid therapy (p = 0.043) and corticosteroid therapy at the time of dismissal (p < 0.001) (), while prolonged OS rates correlated with KPS ≥ 70 (p < 0.001), resection status (p < 0.001) and simultaneous temozolomide therapy (p < 0.001).
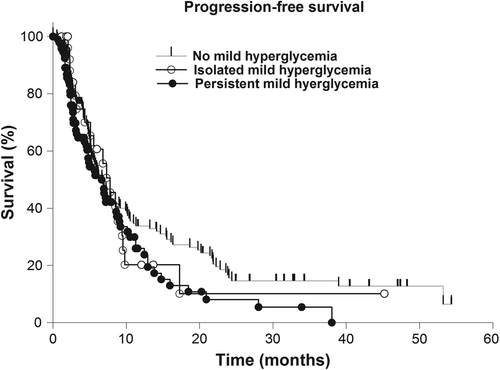
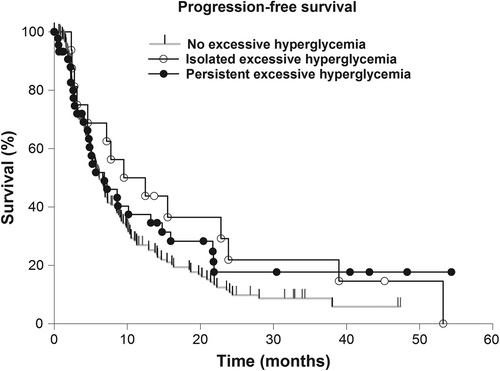
Table I. Univariate proportional-hazards regression analysis of cofactors associated with progression-free and overall survival in glioblastoma patients.
In the univariate survival analysis patients who did not undergo a surgical resection (HR = 1.58; p < 0.001), those with persistent mild hyperglycemia (HR = 2.36; p < 0.001), single excessive hyperglycemic events (HR = 2.57; p = 0.003), and persistent excessive hyperglycemia (HR = 3.48; p ≤ 0.001) were at a significantly higher risk of a more unfavorable clinical course (, and ).
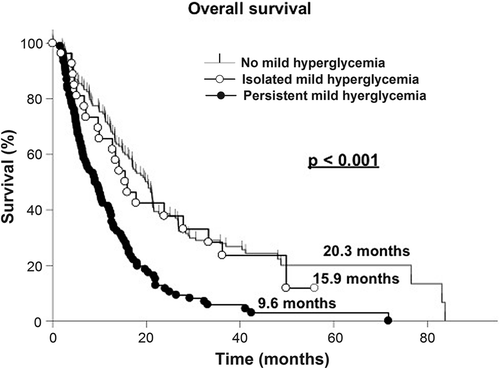
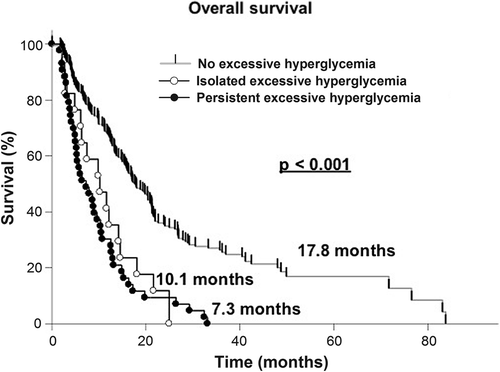
In the multivariate analysis, biopsy (HR = 2.14; p < 0.001), simultaneous temozolomide therapy (HR = 0.73; p < 0.001), corticosteroid therapy at the time of dismissal (HR = 1.55; p = 0.006), persistent mild hyperglycemia (HR = 2.15; p < 0.001) and persistent excessive hyperglycemia (HR = 2.44; p < 0.001) were independently associated with the OS (, and ).
Table II. Multivariate proportional-hazards regression analysis of cofactors associated with overall survival in glioblastoma patients.
Recurrence analysis
The majority of patients showed local progression (n = 124; 66.7%). Recurrence was located distantly and exclusively in the ipsilateral hemisphere in 30 cases (16.1%) and in the contralateral hemisphere in 32 cases (17.2%). Distant recurrence was diagnosed in 59 cases (31.7%) and multifocal recurrence in 62 cases (33.3%). There was no significant influence of persistent mild and excessive hyperglycemia on the likelihood of distant GBM recurrence. Accordingly multifocal progression did not occur more likely in these groups ().
Table III. Odds ratio for the occurrence of multifocal and distant recurrence in glioblastoma patients in regard of hyperglycemia.
Discussion
Currently, there is an ongoing debate concerning the metabolic factors that seem to have an impact on survival in GBM and malignancies in general [Citation18–20].
After analyzing our result for important confounders like corticosteroid therapy, hyperglycemia during radiation therapy remained an independent prognostic factor for GBM survival. Interestingly, corticosteroid therapy at the time of patient dismissal was associated with increased PFS and decreased OS. We could not observe a higher incidence of multifocal (HR: 1,50; p = 0.45) and distant progression (HR: 1,38; p = 0.59) in the small subgroup (n = 12) with persistent excessive hyperglycemia, when compared with the reference group.
High glucose serum levels due to metabolic disorders shift the focus of the interdisciplinary teams fighting the disease of cancer to this potential intrinsic prognostic factor. Tumor cell lines exhibit a three-fold increase in glycolysis for energy generation, compared with their non-malignant counterparts in vitro [Citation21], which underlines the Warburg effect that was first described in the early 20th century [Citation10].
In GBM cells, hyperglycemia showed a promotion of cell growth in vitro. Moreover, a significant incline of apoptosis rates was observed after glucose withdrawal [Citation11]. These findings may be associated with a decreased number of mitochondria per cell, defects in the electron transport pathway of the respiratory chain [Citation22] or a three-fold elevation of glycolysis level in GBM cells [Citation21]. Approaches inhibiting cellular mechanisms controlling the tumors metabolism seem to downregulate tumor cell growth, angiogenesis, and GBM proliferation in vitro [Citation23]. Hence, antidiabetic metformin therapy, activating adenosine monophosphate-activated protein kinase (AMPK), in GBM patients seems to be associated with prolonged survival in glioma patients [Citation24]. It suggests that limiting the energy supplies in tumor cells might be a valuable approach to inhibit glioma progression [Citation24].
Hyperglycemia, as an independent prognostic factor, seems to be associated with decreased survival in cancer in general. Several studies suggest an effect of hyperglycemia on tumor growth, tumor progression, recurrence and mortality in various tumor entities [Citation12,Citation13,Citation25]. Furthermore, retrospective series report on an adverse effect of high glucose levels and survival in GBM patients [Citation15,Citation16,Citation26]. A possible approach to these effects may be the promotion of tumor cell growth, angiogenesis, proliferation and migration in GBM cells caused by high serum glucose [Citation11,Citation23,Citation27]. This theory is supported by different authors suggesting an association between hyperglycemia and tumor progression and recurrence in other entities [Citation13,Citation14].
Mutations on metabolic genes like IDH 1 and 2 seem to promote metabolic deregulations as well as to promote tumor growth and progression [Citation28]. Our study was not able to demonstrate a significant influence of hyperglycemia during chemoradiation on progression patterns in GBM patients.
Thus, we have to consider the potential pathophysiologic influences of hyperglycemia that might result in radioresistance. Glycolytic metabolites, e.g. lactate, could be capable of scavenging free radicals that are mainly responsible for indirect radiation effects [Citation29]. Furthermore high serum glucose levels could have an impact on DNA damage capabilities, as shown previously in bronchial cancer cells [Citation30]. Impact of down-regulated antioxidant systems and the apoptosis protection of hypoxic cells [Citation31] in tumor microenvironment through high glucose serum levels might play a key role in the development of radioresistant properties, that finally result in poorer patient survival and warrants further investigation that will be addressed in upcoming preclinical experiments. In addition, hyperglycemia is associated with hyperinsulinemia, and increased insulin-like growth factor (IGF) expression what may upregulate the tyrosine kinase signaling cascade that may be linked directly to elevated tumor cell proliferation [Citation32].
Valuable information about the long-term control of glucose serum levels might deliver the evaluation of HbA1c in GBM patients during therapy. Nevertheless, the clinical role of diabetes and antidiabetic therapy with metformin on GBM patient survival merit further investigation and will be assessed in an upcoming project at the University Hospital of Heidelberg.
Due to the retrospective nature of this study we are aware that our data do not enable us to draw definitive conclusions on potential pathophysiological mechanisms of hyperglycemia. It is not possible with the current dataset to differentiate if our findings show a direct effect of high glucose levels on pathophysiology or if the results only mirror consequences of an unfavorable subset of patients that experience hyperglycemia. A substantial proportion of cases received corticosteroid therapy at some point during radiation therapy, which might be an explanation of the high occurrence rate of hyperglycemia throughout our patient collective. However, only patients with persistent corticosteroid therapy at the time of dismissal were recorded.
As was to be expected, corticosteroid therapy at dismissal was associated with decreased OS. Obviously, patients with corticosteroid therapy often do not receive a sufficient adjustment of iatrogenic elevated blood glucose levels in clinical routine. The progression-free intervals of patients receiving glucocorticoid therapy after radiation therapy were prolonged. The findings could be related to the decreased clinical symptoms with corticosteroid therapy. This in turn, might lead to an earlier manifestation of clinical symptoms, a shortened follow-up interval, and finally recognition of disease progression at an earlier date in patients who did not receive corticosteroids after dismissal. Current bevacizumab trials could show a diminished rate of corticosteroid use amongst patients with the vascular endothelial growth factor A (VEGF-A) receptor antagonist. These findings were correlated with improved local tumor control and increased quality of life in the bevacizumab group, but could not be translated into prolonged patient survival [Citation33] as seen in our study.
If our findings can be supported by further clinical data, adjusting patient's blood glucose by proper diet and sufficient insulin therapy might represent useful interventions.
This study has several limitations due to its retrospective nature. First, serum glucose level measurements were not standardized and taken randomly in a subset of patients. In these cases patients with known high blood glucose values are likely to be monitored more frequently. This might lead to an overrepresentation of this patient subgroup and represent a possible caveat in data interpretation. Second, patients with progredient tumors, reduced performance status, multifocal disease and intracranial pressure are more likely to receive high-dose corticosteroid therapy during irradiation. Clinical neurologic symptoms, deficits or focal edema are common indications for corticosteroid therapy during radiation therapy, but reasons for this and median corticosteroid doses could not be assessed reasonably in the current study. Third, the number of patients with persistent excessive hyperglycemia and consistent MRI for progression analysis were small (n = 29), which might lead to a bias in the progression analysis. Finally, these factors are associated with reduced survival rates, which might lead to a further bias in the survival analysis with regard to hyperglycemic metabolism.
The strength of this study is the long-term follow-up period, mostly encompassing total survival times. Furthermore, the study comprises over 200 patients and represents one of the largest series published until now. Additionally, survival rates were adjusted for corticosteroid therapy during radiation therapy.
Our data help to understand the influence of hyperglycemia on GBM progression patterns and survival during radiation therapy. To the authors’ knowledge, this report is the first to assess the effect of hyperglycemia on GBM recurrence patterns. Further prospective investigations and in vitro experiments are merited to understand cellular mechanism and objectivize the clinical impact of hyperglycemia and corticosteroid therapy on GBM during radiotherapy.
Conclusion
Our findings are consistent with previous, albeit smaller series, showing that persistent hyperglycemia is an unfavorable predictor of survival in GBM patients during radiation therapy, even when survival rates were adjusted for corticosteroid therapy. However, metabolic misbalances did not show a significant negative impact on GBM relapse patterns. If our findings can be verified in further clinical trials, adjusting patient's blood glucose levels by proper diet and sufficient insulin therapy may be useful approaches.
Declaration of interest: Authors’ disclosures of potential conflicts of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114: 97–109.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96.
- Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 2007;25: 953–64.
- Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L, et al. Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. J Clin Neurosci 2013;20:1422–6.
- Adeberg S, Konig L, Bostel T, Harrabi S, Welzel T, Debus J, et al. Glioblastoma recurrence patterns after radiation therapy with regard to the subventricular zone. Int J Radiat Oncol Biol Phys 2014;90:886–93.
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 2004;79:992–1000.
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. New Engl J Med 2001;345:1359–67.
- Woodworth GF, Chaichana KL, McGirt MJ, Sciubba DM, Jallo GI, Gokaslan Z, et al. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery 2007;61:99–105; discussion -6.
- Warburg O. On the origin of cancer cells. Science 1956; 123:309–14.
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927;8:519–30.
- Jelluma N, Yang X, Stokoe D, Evan GI, Dansen TB, Haas-Kogan DA. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res 2006;4:319–30.
- Suba Z, Barabas J, Szabo G, Takacs D, Ujpal M. Increased prevalence of diabetes and obesity in patients with salivary gland tumors. Diabetes Care 2005;28:228.
- Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: Exploring the hyperinsulinaemia hypothesis. Br J Cancer 2001;84:417–22.
- Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prevent 2002;11:385–91.
- Mayer A, Vaupel P, Struss HG, Giese A, Stockinger M, Schmidberger H. Strong adverse prognostic impact of hyperglycemic episodes during adjuvant chemoradiotherapy of glioblastoma multiforme. Strahlenther Onkol 2014;190: 933–8.
- Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol 2009;27:1082–6.
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203.
- Michaelsen SR, Christensen IJ, Grunnet K, Stockhausen MT, Broholm H, Kosteljanetz M, et al. Clinical variables serve as prognostic factors in a model for survival from glioblastoma multiforme: An observational study of a cohort of consecutive non-selected patients from a single institution. BMC Cancer. 2013;13:402.
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008;134:703–7.
- Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: Connections of metabolism and cell proliferation. Ann NY Acad Sci 2011;1243:54–68.
- Oudard S, Arvelo F, Miccoli L, Apiou F, Dutrillaux AM, Poisson M, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer 1996;74:839–45.
- Lichtor T, Dohrmann GJ. Respiratory patterns in human brain tumors. Neurosurgery 1986;19:896–9.
- Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, et al. Metformin: Taking away the candy for cancer? Eur J Cancer 2010;46:2369–80.
- Welch MR, Grommes C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol 2013;2:237–46.
- Suba Z, Ujpal M. [Correlations of insulin resistance and neoplasms]. Magyar Onkol 2006;50:127–35.
- McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery 2008;63:286–91; discussion 91.
- Guo D, Cloughesy TF, Radu CG, Mischel PS. AMPK: A metabolic checkpoint that regulates the growth of EGFR activated glioblastomas. Cell Cycle 2010;9:211–2.
- Wu W, Zhao S. Metabolic changes in cancer: Beyond the Warburg effect. Acta Biochim Biophys Sin 2013;45: 18–26.
- Sattler UG, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C, et al. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol 2010;94: 102–9.
- Yamamori T, Meike S, Nagane M, Yasui H, Inanami O. ER stress suppresses DNA double-strand break repair and sensitizes tumor cells to ionizing radiation by stimulating proteasomal degradation of Rad51. FEBS Letters 2013; 587:3348–53.
- Vaupel P. Metabolic microenvironment of tumor cells: A key factor in malignant progression. Exp Oncol 2010;32:125–7.
- Boyd DB. Insulin and cancer. Integr Cancer Ther 2003;2: 315–29.
- Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. New Engl J Med 2014;370:2049.
