ABSTRACT
Background. Despite local control now exceeding 90% with image-guided adaptive brachytherapy (IGABT), regional and distant metastases continue to curb survival in locally advanced cervical cancer. As regional lymph nodes often represent first site of metastatic spread, improved nodal control could improve survival. The aim of this study was to examine optimal volume and dose of external beam radiotherapy (EBRT) to maximize regional control including dose contribution from IGABT.
Material and methods. In total 139 patients from the EMBRACE study were analyzed. Individual nodal dose was determined by dose-maps from EBRT and IGABT. All PET/CT scans were re-evaluated and nodal maximal standard uptake value (SUVmax) was determined. Nodal failures were registered to planning scans and related to boosted nodes and treated volume. Relation between SUVmax and nodal control as well as the pattern of regional nodal failure were analyzed.
Results. Eighty-four patients were node positive. Nine patients had all metastatic nodes surgically removed. Seventy-five patients had 209 nodes boosted with EBRT. Median nodal boost dose was 62 Gy EQD2 (53–69 Gy EQD2). Median SUVmax was 6 (2–22). No patients had persistent nodal disease, but six patients recurred in a boosted node. SUVmax was significantly higher in nodes that recurred (p = 0.02). However, there was no correlation to nodal dose or volume. Twenty-one patients had a nodal failure including para-aortic nodal (PAN) metastases above the irradiated volume. Nine patients had a PAN-only failure. Patients receiving ≤ 4 cycles of weekly cisplatin had higher risk of nodal failure (p < 0.01).
Conclusion. Current RT practice provides a high level of control in both boosted nodes and the elective irradiated regional target. However, a high nodal SUVmax is a negative prognostic predictor for nodal control. Attention should be raised to administration of a complete schedule of concurrent chemotherapy as well as treatment of para-aortic nodes.
Standard treatment for locally advanced cervical cancer consists of external beam radiotherapy (EBRT), concurrent chemotherapy and brachytherapy (BT) [Citation1]. During the last decade, advances in the treatment of the primary tumor have been achieved. In EBRT adaptive strategies are being investigated to secure coverage of the uterus [Citation2]. Magnetic resonance imaging (MRI) has been introduced in the planning of image-guided adaptive brachytherapy (IGABT) [Citation3–5] and target volume delineation has been investigated [Citation6,Citation7]. The implementation of IGABT in many centers has resulted in local control rates > 90% leading to an increase in survival by about 10% [Citation8–10]. However, regional nodal control remains a major hindrance for further improvement in survival.
Approximately 50% of all patients with locally advanced cervical cancer have regional lymph node metastases at diagnosis [Citation11,Citation12]. Metastatic spread to regional lymph nodes or nodal failure after treatment are well described negative prognostic factors for survival [Citation13]. The optimal treatment strategy for obtaining regional nodal control is still under investigation [Citation14]. Possible micro-metastatic disease is usually dealt with by electively irradiating all pelvic nodal stations including sometimes also the para-aortic region with EBRT to 45–50 Gy. Metastatic nodes themselves can be treated either by surgery or chemo-radiotherapy with a boost of EBRT delivered to the pathological node. Nodal boost can be delivered as a sequential or simultaneous integrated boost (SIB). With a sequential boost additional dose is most often delivered with narrow parallel-opposed fields. With the use of intensity-modulated radiotherapy (IMRT) different doses to different parts of the target volume can be delivered giving a SIB to pathological nodes [Citation15]. Many centers deliver a nodal dose of 60 Gy [Citation14]. Recent studies evaluating the dose delivered by EBRT to metastatic nodes suggests that even a dose of 55 Gy/25 fractions is sufficient to control nodes up to a nodal diameter of 2–3 cm [Citation16,Citation17].
Few studies have investigated dose response for larger nodes. To our knowledge no previous studies have reported the total nodal dose to boosted nodes incorporating the contribution from BT. Recent studies have demonstrated that BT contributes with 1.6–6.2 Gy EQD2 to pelvic nodes depending on the treatment technique and nodal location [Citation18,Citation19]. Hence, the BT contribution should be considered when prescribing dose to metastatic pelvic nodes.
Nodal failure within a boosted node may be due to insufficient dose whereas nodal failure within the elective irradiated volume or outside likely is due to the limited ability for diagnosing micro metastases. Several studies have pointed to the para-aortic nodes (PAN) as a region in which nodes often recur [Citation20,Citation21]. In fact, PAN failure might be caused by false negative imaging at diagnosis as demonstrated by recent studies comparing the results of laparoscopic PAN staging with PET/CT [Citation22,Citation23].
The aim of the present study was to determine the total dose of EBRT and IGABT delivered to regional metastatic nodes and to investigate the relationship between nodal control and nodal dose. Furthermore, the effect of nodal volume and nodal SUVmax on nodal outcome was investigated. Finally, an analysis of the pattern of regional nodal failures was investigated within both node negative and node positive patients with special focus on PAN failures.
Materials and methods
Patients and diagnostic work-up
The study includes 139 patients accrued from March 2008 to March 2013 to the EMBRACE study (www.embracestudy.dk) from either Aarhus University Hospital (AUH) or University Medical Center Utrecht (UMCU). Pre-treatment evaluation consisted of patient's history, clinical exam including staging in general anesthesia (FIGO), blood samples, biopsy from the primary tumor and MRI scan of the pelvis and abdomen. For patients treated at AUH an additional FDG positron emission tomography (PET)/computed tomography (CT) scan was performed for all patients. For patients treated at UMCU a FDG PET or a FDG PET/CT scan was added if the MRI showed suspicious lymph nodes. PET/CT studies were performed according to local scanning protocols. PET/CT scans were acquired 60–90 minutes after injection of a standard dose of approximately 370 MBq 18F-FDG. Blood glucose levels were measured routinely or only in patients with known diabetes depending on local practice.
Lymph nodes were considered pathologic if the SUV value was above blood background or if they measured > 10 mm on short axis on MRI or CT. Nodes between 5 and 10 mm without FDG uptake were considered pathologic if they had an irregular shape, had lost their nodal architecture, were inhomogeneous or had an irregular border on MRI. All PET scans were re-evaluated for the present study by an oncologist and a specialist in nuclear medicine and SUVmax values for the pathologic nodes were recorded.
Treatment
RT schedules for the two centers are summarized in . In both institutions treatment consisted of EBRT, concomitant weekly cisplatin (40 mg/m2) and IGABT delivered either as pulsed-dose rate (PDR) or high-dose rate (HDR) in the final weeks of the treatment. The intended maximal overall treatment time was 50 days including IGABT. The planning aim of EBRT+ IGABT was to deliver at least 85 Gy (EQD2) to D90 of the high-risk clinical target volume (HR-CTV). EBRT to the elective nodal stations (CTV-E) was delivered as either three-dimensional conformal radiotherapy (3D-CRT) or IMRT. The CTV-E upper border was either at the L4/L5 interspace or at the level of the aortic bifurcation. If the patients had nodal involvement at the level of the common iliac artery or higher the CTV-E was expanded to include the PAN region with the upper border at the L1/L2 interspace. A few elderly node negative patients were treated with the CTV-E border at the L5/S1 interspace. Pathologic lymph nodes (GTV-N) were boosted with either a SIB or a sequential boost. Laparoscopic nodal staging was not routinely performed. However, at UMCU nodes larger than 2 cm were surgically removed if possible whereas at AUH all pathological nodes were boosted with EBRT using SIB regardless of size.
Table I. Treatment schedules of external beam radiotherapy (EBRT) and brachytherapy (BT) of locally advanced cervical cancer at University Medical Center Utrecht and Aarhus University Hospital. HDR, high-dose rate; PDR, pulsed-dose rate.
Determination of total nodal dose
For each patient diagnostic MRI and PET/CT scans, as well as MRI datasets at time of BT were rigidly registered to the planning CT scan using a bone match. In case of nodal failure, MRI and PET/CT data sets at time of failure were also registered to the CT scan. To investigate the delivered dose to each node EBRT dose maps (elective and boost) as well as the BT dose maps were linked to the planning CT. Gross tumor volume for each node (GTV-N) was contoured on the master planning CT scan using information from MRI and PET/CT scans. Contouring was performed with a customized software package VolumeTool that offers a 3D presentation of all datasets [Citation24].
The physical delivered dose to 98% (D98) of each GTV-N was determined using the dose maps of EBRT elective, EBRT boost and BT. The biologically equivalent dose in 2 Gy fractions (EQD2) was calculated, using the linear quadratic model with α/β = 10 for tumor and a repair half-time of 1.5 hours, and the total dose was determined.
Follow-up
All patients were followed prospectively with a gynecologic examination every three months the first year, every six months for year 2–3 and annually for year 4 and 5. MRI was performed routinely three and 12 months after completed treatment. For 82 patients an additional routine PET/CT was performed three months after completed therapy. Additional imaging was performed on clinical indication. Local failure was defined as persistent disease or recurrence of the primary tumor. When a nodal failure was diagnosed the relation to previously boosted nodes and treatment field was determined from the failure scan. Distant failure was defined as a failure higher than the PAN area, i.e. above the L1-L2 interspace.
Statistical analysis
Data were analyzed using Stata statistical software, version 13.0. Follow-up time and time to failure were calculated from the end of treatment date. Patients were censured at time of failure, death or last follow-up visit. Local control rate, nodal control rate and overall survival were calculated using Kaplan-Meier statistics. The Wilcoxon rank sum test was used to test for nodal dose, volume and SUVmax in relation to failure. A χ2-test was used to test for chemotherapy and pathology in relation to nodal failure. Statistical significance was defined as a p-value < 0.05.
Results
Patient characteristics are shown in . Briefly, 106 patients (77%) had squamous cell carcinoma, 111 (80%) were FIGO stage II-IV and 84 (60%) were node positive. Seven node positive patients with stage IB disease were treated with total lymphadenectomy, but leaving the primary tumor in situ for definitive chemo-radiotherapy. At UMCU two patients had all suspicious nodes removed before RT. The remaining 75 patients had a total of 209 nodes boosted. The majority of the nodes were located at the external or internal iliac artery. Twenty-five patients had lymph nodes at the level of common iliac (CI) artery or higher and were treated with an extended elective field including PAN. shows the location of involved nodes at diagnosis and at time of failure.
Table II. Patient and tumor characteristics for node positive and node negative patients with locally advanced cervical cancer.
Table III. Location of nodal metastases at time of diagnosis (84 patients) and at time of failure (21 patients). In some patients nodal metastases and nodal failures was diagnosed in more than one region.
The median follow-up time was 30 months (range 3–64 months). At three years the actuarial overall survival was 79% (95% CI 70–85%), regional nodal control was 83% (95% CI 75–89%) and local control was 93% (95% CI 86–96%). During the follow-up period 41 patients failed locally, regionally or systemically. The locations of all failures are shown in . Nine patients had a local failure (5%) and four of these had persistent disease at first follow-up after treatment. Seventeen patients had distant failure (12%). Distant failures were mainly in the lung or in lymph nodes above the L1-L2 interspace.
Figure 1. Location of all failures (41 patients) and nodal failures (21 patients). Overlap is due to patients failing in more than one region. Local failures are here defined as persistent disease or recurrence of the primary tumor. Nodal failures consist both of failures within the electively irradiated volumes as well as non-irradiated para-aortic failures. Distant failures do not included para-aortic failures below L1.
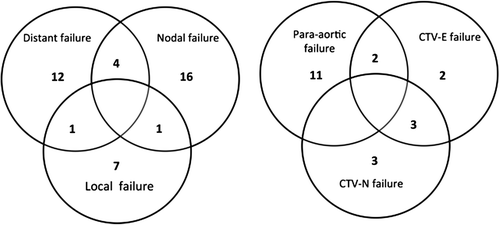
Twenty-one patients (15%) had a nodal failure including PAN metastases. Thirteen patients had PAN failure above the irradiated volume. Two had simultaneous failure in the elective field and two and simultaneous distant failures. The remaining nine patients had PAN metastases as the only site of failure. Only one of these patients was initially treated with an extended field due to PAN metastases, the remaining eight had a CTV-E border at the L5/SI interspace (n = 2), L4/L5 interspace (n = 5) or aortic bifurcation (n = 1). Three out of the nine patients were node negative at diagnosis. For the five node positive patients treated with a pelvic field the highest level of nodal involvement at diagnosis was at the internal/external iliac artery. The median time to isolated PAN failure was 6.6 months (range 2.5–28.5 months). Four patients had isolated PAN failure diagnosed on the first follow-up imaging.
The median number of cisplatin cycles was six (range 0–7). Thirty-one patients received ≤ 4 cycles of weekly cisplatin due to advanced age, chronic diseases or low white blood cell count. A higher incidence of nodal failure was found in patients receiving less than five courses of cisplatin (p < 0.01). No significant relation between pathology (adenocarcinoma + adeno-squamous versus squamous) and nodal failure was found.
The median number of boosted nodes per patient was two (range 1–10). The median volume of boosted nodes was 1.5 cm3 (range 0.1–44.9 cm3). Median dose delivered to all nodes was 62.4 Gy EQD2 (range 52.9–69.1 Gy EQD2). The median dose contribution from BT was 3.1 Gy EQD2 (range 0–12.8 Gy EQD2). No patients had persistent disease in the boosted nodes, but six patients had a later failure within such a node. The median time to failure for these patients was 18.8 months (range 12.8–32.5 months). The median dose to recurrent nodes was 62.9 Gy EQD2 (range 59–68.1 Gy EQD2) and the median nodal volume at diagnosis 2.3 cm3 (range 1.2–9.2 cm3). There was no significant correlation for failure with neither dose nor volume. The dose and volume of all boosted nodes are presented in .
Figure 2. Panel A shows the nodal dose and nodal volume for controlled (white) and failed nodes (black). All nodes were considered pathologic at diagnosis. Panel B shows the nodal dose and nodal SUVmax for controlled (white) and failed nodes (black).
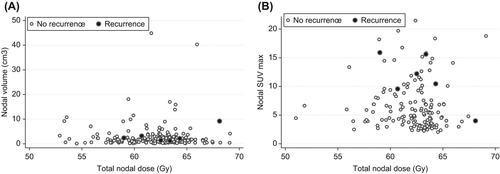
One hundred and twelve patients had a diagnostic PET or PET/CT. In total 144 nodes had a standard uptake value (SUV) above blood background and were considered pathologic. The median SUVmax of all nodes was 5 (range 2–21). For the six recurrent nodes the median SUVmax was 11 (range 4–16). Nodal SUVmax was significantly higher for the recurrent nodes (p = 0.02). The dose and nodal SUVmax are presented in .
Discussion
In our study we demonstrated a high nodal control rate in boosted lymph nodes with failure in six of 209 initially diagnosed nodes. There is currently no consensus on appropriate dose levels for lymph node boosting. Three recent studies applied different dose levels and investigated the control of regional lymph node metastases in locally advanced cervical cancer. In these studies nodal malignancy was determined by imaging (MRI and/or PET/CT) but no histological verification was performed. Vargo et al. explored the outcome in lymph node positive patients by delivering a simultaneously integrated lymph node boost of 55 Gy/25 fractions [Citation16]. Extended elective field irradiation including the PAN area combined with a boost to pathologic nodes was applied in all patients. Excellent nodal control was obtained with only 1/61 patients recurring in a boosted node. Ariga et al. investigated EBRT boost prescribing a median dose of 56 Gy to metastatic nodes. In that study 5/57 patients recurred in boosted nodes [Citation21]. In 2004 Grigsby et al. investigated different boost doses according to nodal size. In that study a median dose of 67.2 Gy was delivered. Only 5/132 patients recurred within a boosted node [Citation25]. Neither of the studies by Grigsby et al. or Ariga et al. found a significant relation between nodal size and failure.
To our knowledge, the present study is the largest study to date determining the exact nodal dose delivered by incorporating the dose contribution from BT. The study by Van den Bos et al. indicated that BT contributed with 1.6–6.2 Gy EQD2 to the total nodal dose depending on treatment technique and location of the node [Citation19]. Recently, a study by Mohamed et al. demonstrated that BT contributes with a mean D50 dose of 3.8–6.2 Gy EQD2 to the different pelvic node groups [Citation18]. A similar BT contribution was seen in the current study with a median dose of 3.1 Gy EQD2 (range 0–12.8 Gy EQD2) and BT contribution should therefore be taken into account when prescribing the total nodal dose. The median total EBRT and BT dose of 62.4 Gy EQD2 gave a high nodal control with only six of 75 patients recurring within a boosted node. As in the studies by Ariga et al. and Grigsby et al., it was not possible to identify any impact of nodal size on nodal control. Due to different EBRT boost dose levels at UMCU and AUH and due to the heterogeneity of BT dose, the patients in this study received a wide range of dose between 53 and 69 Gy EQD2. Our data did not indicate any improved lymph node control with higher boost doses. Hence there seems to be no indication of a general benefit of delivering a total lymph node dose beyond 60 Gy EQD2.
Concurrent use of chemotherapy has been used as standard treatment since 1999. In 2008 a meta-analyses of 18 studies demonstrated a benefit of chemotherapy not only on OS but also on local and distal control [Citation26]. The importance of concurrent chemotherapy is underlined in our study, where a significant relation between nodal failures and the number of chemotherapy courses was established.
With the use of FDG PET/CT additional prognostic information may be obtained for both the primary tumor and nodal metastases. Kidd et al. demonstrated that nodes with a SUVmax > 4.3 had a significantly higher risk of persistent disease or failure [Citation13]. Onal et al. showed that a nodal SUVmax > 7.5 resulted in a decreased disease-free survival and OS, but could only show a non-significant trend for a higher risk of failure within the boosted nodes with SUVmax > 7.5 (p = 0.12) [Citation27]. In our study, there was a significant correlation between SUVmax and failure in boosted nodes. SUVmax was a stronger negative prognostic factor for nodal failure than size or volume of lymph nodes, and dose escalation or surgical removal of lymph nodes may be particularly relevant in patients with high nodal SUVmax. The limitations inherent to all multi-center studies involving semi-quantitative 18F-FDG PET also apply to this study. Measured SUV values may thus in extreme cases vary up to 50% between different scanner systems and reconstruction algorithms. However, both participating PET centres have decades of experience in conducting PET research and conform to the guidelines as laid out by the European Association of Nuclear Medicine (EANM) [Citation28]. Scanners at both centers are routinely calibrated using standard phantoms and reconstruction algorithms, which are also applied to clinical studies. We are therefore confidant that our 18F-FDG PET images are comparable and that SUV values can safely be reported.
PET/CT has a higher detection rate of PAN metastases compared to CT and MRI but still has a false negative rate of 5–17% [Citation22]. Due to this fact some PAN metastases might not be detected and irradiated. PAN was the most frequent site of nodal failure in our study, with 13 patients recurring above the treatment field. Beadle et al. have previously reported similar observations [Citation20]. Only two of the 13 patients were treated with an extended field due to PAN metastases at diagnosis, and the rest was treated with a pelvic-only field. In our study four patients recurred with PAN metastases within the first three months after treatment and thus probably had undetected PAN metastases at diagnosis. Hence, there is still room for improving detection and treatment of PAN disease. Current approaches include extended field chemo-radiotherapy, para-aortic surgical staging, and adjuvant chemotherapy.
Vargo and colleagues reported on the outcome and toxicity following extended fields to all patients with evidence of pelvic or PAN nodal disease on PET/CT [Citation16]. Only two patients recurred in the PAN area. Late grade 3–4 toxicity was reported to be low. One patient had a grade 4 recto-vaginal fistula, and no ≥ grade 3 late bladder, hematologic or small bowel toxicity was reported. In the current study extended field was delivered in patients with nodal metastases to CI or PAN nodes. Based on the Vargo study and on the patterns of spread observed in our patient cohort, more patients might benefit from elective PAN irradiation and focus should be raised to detect these patients. Several risk criteria will be applied to select patients for elective PAN irradiation in the forthcoming EMBRACE II study. This strategy will increase the number of patients treated with an extended field and hopefully subsequently decrease the number of PAN failures.
Gouy et al. investigated the overall survival after laparoscopic PAN staging and tailored treatment with elective irradiation of the PAN area in patients with positive PAN nodes. In that study a comparable survival rate of patients with PAN micro metastases (< 5 mm) and patients without PAN disease was demonstrated [Citation22]. Based on this knowledge, a phase III clinical trial has recently started recruiting patients with positive pelvic lymph nodes and negative para-aortic nodes on PET/CT. Patients are randomized between pelvic chemo-radiation or laparoscopic para-aortic staging with chemo-radiation with expansion of the field according to metastatic status of the PAN nodes. The study expects to show a 9% benefit in overall survival at three years [Citation29].
In the ongoing phase III OUTBACK study (NCT01414608) the effect of adjuvant chemotherapy after chemo-radiation is investigated. Possibly this study will demonstrate that the use of adjuvant chemotherapy can secure a higher control of undetected PAN metastases.
In conclusion, current treatment schedules secure high nodal control in both metastatic boosted nodes and the irradiated elective nodal region in patients with locally advanced cervical cancer. A dose response relationship for nodal control could not be established due to the limited number of events, but our results do not indicate a general benefit of increasing the lymph node dose beyond 60 Gy EQD2 total EBRT and BT dose. However, nodal SUVmax is a negative prognostic predictor for nodal control, and further intensification of treatment of lymph nodes with a high SUVmax could be considered. Attention should also be raised to administration of a complete schedule of concurrent chemotherapy as well as treatment of para-aortic nodes.
Declaration of interest: This work was supported by the Danish Cancer Society and by CIRRO – The Lundbeck Foundation Center for Investigational Research in Radiation Oncology. The authors report no conflict of interests.
References
- Barbera L, Thomas G. Management of early and locally advanced cervical cancer. Semin Oncol 2009;36:155–69.
- Ahmad R, Bondar L, Voet P, Mens JW, Quint S, Dhawtal G, et al. A margin-of-the-day online adaptive intensity- modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: A dosimetric evaluation. Acta Oncol 2013; 52:1430–6.
- Fokdal L, Tanderup K, Hokland SB, Rohl L, Pedersen EM, Nielsen SK, et al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol 2013;107:63–8.
- Schmid MP, Fidarova E, Potter R, Petric P, Bauer V, Woehs V, et al. Magnetic resonance imaging for assessment of parametrial tumour spread and regression patterns in adaptive cervix cancer radiotherapy. Acta Oncol 2013;52: 1384–90.
- Palmqvist T, Dybdahl Wanderas A, Langeland Marthinsen AB, Sundset M, Langdal I, Danielsen S, et al. Dosimetric evaluation of manually and inversely optimized treatment planning for high dose rate brachytherapy of cervical cancer. Acta Oncol 2014;53:1012–8.
- Hegazy N, Potter R, Kirisits C, Berger D, Federico M, Sturdza A, et al. High-risk clinical target volume delineation in CT-guided cervical cancer brachytherapy: Impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol 2013;52:1345–52.
- Petric P, Hudej R, Rogelj P, Blas M, Tanderup K, Fidarova E, et al. Uncertainties of target volume delineation in MRI guided adaptive brachytherapy of cervix cancer: A multi-institutional study. Radiother Oncol 2013;107:6–12.
- Lindegaard JC, Fokdal LU, Nielsen SK, Juul-Christensen J, Tanderup K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52:1510–9.
- Nomden CN, de Leeuw AA, Roesink JM, Tersteeg RJ, Moerland MA, Witteveen PO, et al. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: A single institution experience. Radiother Oncol 2013;107:69–74.
- Potter R, Georg P, Dimopoulos JC, Grimm M, Berger D, Nesvacil N, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23.
- Cosin JA, Fowler JM, Chen MD, Paley PJ, Carson LF, Twiggs LB. Pretreatment surgical staging of patients with cervical carcinoma: The case for lymph node debulking. Cancer 1998;82:2241–8.
- Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: Relationship to prognosis. J Clin Oncol 2010;28:2108–13.
- Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer 2010;116:1469–75.
- Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, et al. ACR Appropriateness Criteria® on Advanced Cervical Cancer Expert Panel on radiation oncology-gynecology. Int J Radiat Oncol Biol Phys 2011;81:609–14.
- Cihoric N, Tapia C, Kruger K, Aebersold DM, Klaeser B, Lossl K. IMRT with (1)(8)FDG-PET\CT based simultaneous integrated boost for treatment of nodal positive cervical cancer. Radiat Oncol 2014;9:83–717X–9–83.
- Vargo JA, Kim H, Choi S, Sukumvanich P, Olawaiye AB, Kelley JL et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys 2014;90:1091–8.
- Rash DL, Lee YC, Kashefi A, Durbin-Johnson B, Mathai M, Valicenti R, et al. Clinical response of pelvic and para-aortic lymphadenopathy to a radiation boost in the definitive management of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2013;87:317–22.
- Mohamed SM, Aagaard T, Fokdal LU, Pedersen EM, Lindegaard JC, Tanderup K. Assessment of radiation doses to the para-aortic, pelvic, and inguinal lymph nodes delivered by image-guided adaptive brachytherapy in locally advanced cervical cancer. Brachytherapy 2015;14:56–61.
- van den Bos W, Beriwal S, Velema L, de Leeuw AA, Nomden CN, Jurgenliemk-Schulz IM. Image guided adaptive brachytherapy for cervical cancer: Dose contribution to involved pelvic nodes in two cancer centers. J Contemp Brachytherapy 2014;6:21–7.
- Beadle BM, Jhingran A, Yom SS, Ramirez PT, Eifel PJ. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2010;76:1396–403.
- Ariga T, Toita T, Kasuya G, Nagai Y, Inamine M, Kudaka W, et al. External beam boost irradiation for clinically positive pelvic nodes in patients with uterine cervical cancer. J Radiat Res 2013;54:690–6.
- Gouy S, Morice P, Narducci F, Uzan C, Martinez A, Rey A, et al. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol 2013;31:3026–33.
- Ramirez PT, Jhingran A, Macapinlac HA, Euscher ED, Munsell MF, Coleman RL et al. Laparoscopic extraperitoneal para-aortic lymphadenectomy in locally advanced cervical cancer: A prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer 2011;117:1928–34.
- Bol GH, Kotte AN, van der Heide UA, Lagendijk JJ. Simultaneous multi-modality ROI delineation in clinical practice. Comput Methods Programs Biomed 2009;96:133–40.
- Grigsby PW, Singh AK, Siegel BA, Dehdashti F, Rader J, Zoberi I. Lymph node control in cervical cancer. Int J Radiat Oncol Biol Phys 2004;59:706–12.
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802–12.
- Onal C, Guler OC, Reyhan M, Yapar AF. Prognostic value of (18)F-fluorodeoxyglucose uptake in pelvic lymph nodes in patients with cervical cancer treated with definitive chemoradiotherapy. Gynecol Oncol 2015;137:40–6.
- Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328–54.
- Frumovitz M, Querleu D, Gil-Moreno A, Morice P, Jhingran A, Munsell MF, et al. Lymphadenectomy in locally advanced cervical cancer study (LiLACS): Phase III clinical trial comparing surgical with radiologic staging in patients with stages IB2-IVA cervical cancer. J Minim Invasive Gynecol 2014;21:3–8.
