ABSTRACT
Background. Intrafraction motion may compromise the target dose in stereotactic body radiation therapy (SBRT) of tumors in the liver. Respiratory gating can improve the treatment delivery, but gating based on an external surrogate signal may be inaccurate. This is the first paper reporting on respiratory gating based on internal electromagnetic monitoring during liver SBRT.
Material and methods. Two patients with solitary liver metastases were treated with respiratory-gated SBRT guided by three implanted electromagnetic transponders. The treatment was delivered in end-exhale with beam-on when the centroid of the three transponders deviated less than 3 mm [left-right (LR) and anterior-posterior (AP) directions] and 4mm [cranio-caudal (CC)] from the planned position. For each treatment fraction, log files were used to determine the transponder motion during beam-on in the actual gated treatments and in simulated treatments without gating. The motion was used to reconstruct the dose to the clinical target volume (CTV) with and without gating. The reduction in D95 (minimum dose to 95% of the CTV) relative to the plan was calculated for both treatment courses.
Results. With gating the maximum course mean (standard deviation) geometrical error in any direction was 1.2 mm (1.8 mm). Without gating the course mean error would mainly increase for Patient 1 [to -2.8 mm (1.6 mm) (LR), 7.1 mm (5.8 mm) (CC), −2.6 mm (2.8mm) (AP)] due to a large systematic cranial baseline drift at each fraction. The errors without gating increased only slightly for Patient 2. The reduction in CTV D95 was 0.5% (gating) and 12.1% (non-gating) for Patient 1 and 0.3% (gating) and 1.7% (non-gating) for Patient 2. The mean duty cycle was 55%.
Conclusion. Respiratory gating based on internal electromagnetic motion monitoring was performed for two liver SBRT patients. The gating added robustness to the dose delivery and ensured a high CTV dose even in the presence of large intrafraction motion.
Stereotactic body radiation therapy (SBRT) with tightly conformed target dose distributions, steep dose gradients, and high fraction doses is highly efficient in therapy of primary and secondary liver tumors [Citation1–6]. Accurate dose delivery is crucial to ensure tumor coverage and healthy tissue sparing [Citation7]. However, for liver SBRT the treatment accuracy is challenged by tumor motion during treatment delivery, which can exceed several centimeters [Citation8,Citation9] and may lead to target doses that deviate substantially from the planned dose distribution to a static target [Citation10].
The detrimental dose effects of the tumor motion may be mitigated by respiratory gating [Citation11,Citation12]. So far, gating in liver SBRT has mainly relied on external surrogate monitoring rather than direct tumor motion monitoring [Citation13–15] although the external monitoring can be inaccurate [Citation16,Citation17] and studies have emphasized the need for direct internal monitoring [Citation14]. The Real-time Tumor-tracking Radiation Therapy (RTRT) system has been applied for respiratory gating during liver SBRT based on real-time localization of implanted gold markers by stereoscopic x-ray fluoroscopy [Citation18], but this imaging system is not readily available and the x-ray imaging adds ionizing radiation to the patient.
As an alternative, an internal real-time position signal may be provided by implanted electromagnetic transponders, such as the Calypso system, which has been used extensively in the prostate [Citation19] and was recently CE marked and cleared by the US Food and Drug Administration for general implantation in soft tissues. The first use of Calypso in a liver patient took place at University of Louisville in March 2015 [Citation20]. Phantom studies have demonstrated high dosimetric accuracy of Calypso-guided gating [Citation21], but to our knowledge no results on the accuracy in a clinical setting have been published so far. In this paper on our first experiences, we report on the feasibility and the geometric and dosimetric accuracy for the two first liver SBRT patients treated with Calypso-guided respiratory gating at Aarhus University Hospital.
Material and methods
Patients, planning, and treatments
This paper reports on the first two patients treated with Calypso-guided respiratory gating at Aarhus University Hospital. After providing informed consent, the patients received liver SBRT in 3 fractions in the period 16–23 April 2015 following an approved clinical trial protocol on Calypso-gated liver SBRT. Patient 1 was a 76-year-old female treated for a solitary liver metastasis [clinical target volume (CTV): 74 cm3] from breast cancer. Patient 2 was a 77-year-old male treated for a solitary liver metastasis from colorectal cancer (CTV: 26 cm3). Guided by ultrasound, each patient had three electromagnetic transponders implanted percutaneously with 14G needles in close proximity to the metastasis (Calypso Soft Tissue Beacon Transponders, Varian Medical Systems, Palo Alto, CA, USA). A four-dimensional computed tomography (4DCT) scan and a breath-hold end-exhale CT scan, both with 2 mm slice thickness, were acquired the day after transponder implantation. The end-exhale scan was acquired with intravenous contrast (150-mL Visipaque 270, GE Healthcare, Little Chalfont, UK). For Patient 2, target delineation and planning were performed on the contrast-enhanced end-exhale scan. Patient 1 was not able to hold breath throughout the end-exhale scan. For this patient, the end-exhale phase of the 4DCT was used for planning as this was a better representative for the internal anatomy at the end-exhale position during free breathing. An in-house developed frame with a vacuum cushion was used for patient immobilization. No abdominal compression was used.
The planning target volume (PTV) was formed by expanding the CTV by 5 mm margins in the left-right (LR) and anterior-posterior (AP) directions and 10 mm in the cranio-caudal (CC) direction. Both patients had a seven-field conformal plan with a CTV mean dose of 100% of the prescription dose and minimum target doses of 95% to the CTV and 67% to the PTV, i.e. a non-uniform PTV dose distribution with 50% higher dose in the central part [Citation22,Citation23]. Following a risk-adapted strategy, fraction doses of 18.75 Gy (Patient 1) and 20.6 Gy (Patient 2) were prescribed to the 100% dose level. Each transponder was delineated, and its center-of-mass position with respect to the isocenter was recorded.
The respiratory-gated treatments were delivered in free breathing with a TrueBeam accelerator (version 2.0) equipped with a Calypso 3.0 system (Varian Medical Systems). The dose rate was 600 monitor units (MU) per minute. The transponder positions were recorded at 25 Hz by the Calypso system, and the treatment beam was gated off when the centroid position of the three transponders deviated from the planned position by more than 3 mm in the LR or AP direction or more than 4 mm in the CC direction. After patient positioning the treatment couch was adjusted such that the target position reported by Calypso was inside the gating window at end exhale and had a mean value of approximately zero in all three directions during the open gate time intervals. A cone beam (CB) CT scan was acquired in this position and registered to the exhale planning CT scan in order to confirm that the Calypso transponders were close to their planned positions at exhale and that the spinal cord was less than 10 mm from its planned position [corresponding to the planning organ at risk volume (PRV)]. No couch shift was performed based on the CBCT scan, but the couch was adjusted between treatment beams if baseline drift caused misalignment of more than 2 mm between the end exhale position and the gating window.
Data analysis
Two types of log files were retrieved after each treatment: 1) Calypso log files with the recorded transponder motion, an approximate beam-on flag based on a radiation monitor, and the performed couch shifts; and 2) TrueBeam trajectory log files with the delivered MU and the accelerator beam-hold status recorded every 20 milliseconds. The two log files were synchronized by temporal alignment of accelerator beam-holds with Calypso recorded target motion outside of the gating window. The synchronization was used to determine the target motion during beam delivery in the open-gate periods. Note that the temporal alignment neglected beam gating latencies and therefore resulted in small overestimations of the open-gate target displacement when the gate turned on and underestimations of the displacement when the gate turned off.
For comparison, non-gated treatment delivery was simulated for each treatment fraction in order to estimate the would-be intra-treatment target motion during beam-on without gating. First, the recorded motion relative to the isocenter was corrected for any inter-field couch shifts in order to obtain the internal target motion within the patient as it would have been without intervention during treatment. The corrected motion curve was then offset to a mean position of zero during the 60 seconds CBCT scan in order to mimic a CBCT setup with alignment of the target to its mean position during the setup CBCT scan and no further imaging during the treatment session. It reflects our current standard liver SBRT treatments guided by implanted gold fiducial markers. For Patient 2, the Calypso signal was not available during the CBCT scan because lateral centering of the couch during the scan moved the transponders outside of the Calypso localization volume. For this patient, the recorded motion in the last 60 seconds before the couch centering was assumed to represent the motion during CBCT scanning. In the non-gated treatment simulations, we assumed that each treatment beam was delivered without interruption with a constant dose rate of 600 MU/minute and with the same starting time relative to the motion trajectory as in the actual gated treatments. For each fraction, the mean and standard deviation of the intra-treatment target position was calculated for both the actual gated treatment and the simulated non-gated treatment.
The dosimetric impact of the motion was estimated by reconstruction of the delivered CTV dose by an experimentally validated method [Citation24,Citation25] that models the motion of a rigid target as multiple isocenter shifts in an in-house built software program (Matlab) and utilizes the clinical treatment planning system for the dose calculation [Eclipse 11.0, Anisotropic Analytical Algorithm (AAA), Varian Medical Systems]. A 1.5 mm bin width was used, i.e. all target positions within a 1.5 × 1.5 × 1.5 mm3 cube were given the same isocenter shift. For the first fraction and for the entire treatment course consisting of three fractions, the CTV mean dose (Dmean) and the minimum dose to 95% and 99% of the CTV (D95 and D99) with and without gating were calculated and compared with the planned doses.
Results
Three Calypso transponders were implanted within close proximity of the target lesion in the liver in both patients without complication and with only minor pain related to the procedure.
shows the internal target motion at fraction 2 for each patient. A gradual baseline drift for Patient 1 resulted in an internal mean position during the last field that was shifted 3.5 mm to the right, 11.4 mm cranially, and 3.9 mm posteriorly relative to the mean position during the CBCT scan (, left). Three couch corrections were performed during this fraction to compensate for the baseline drift (, left). A similar baseline drift also occurred during fractions 1 and 3 for this patient as seen by the large mean errors in simulated treatments without gating in . Although Patient 2 had a much more stable baseline position there was still a tendency to cranial drift that resulted in a mean position during the last field that was 5.3 mm (fraction 1), 2.5 mm (fraction 2), and 3.0 mm (fraction 3) more cranial than the mean position during the CBCT scan.
Figure 1. Calypso-recorded liver tumor motion at fraction 2 for both patients in the left-right (LR), cranio-caudal (CC), and anterior-posterior (AP) directions. The motion has been corrected for couch shifts, which were performed at the times indicated by vertical red lines. Shaded areas indicate the time for the CBCT scanning (dark gray, Patient 1), simulated CBCT scanning (dark gray, Patient 2), and beam delivery (light gray). Beam pauses due to gating are not shown. The Calypso signal was unavailable while the couch was rotated between the third and second last beam for Patient 1 and during the actual CBCT scan for Patient 2 due to lateral couch centering.
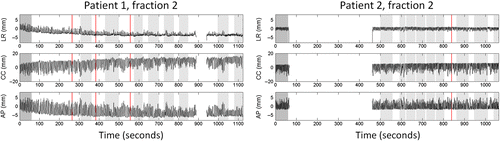
Table I. Treatment data for each fraction (Fx) for the two patients (Pt), including number of couch shifts, time from CBCT scan to start of the first field (Tstart), time between start of the first field and end of the last field (Ttreatment), and the mean error during beam-on in the actual treatments with respiratory gating and in simulated treatments without gating.
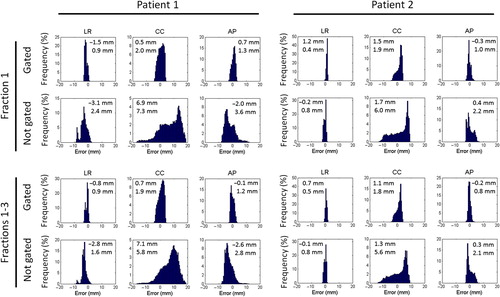
The duty cycle, i.e. the fraction of the gated field delivery time with beam on, was in mean 54% for Patient 1 and 55% for Patient 2 (). The mean time between the CBCT scan and treatment start and the mean treatment duration were 9.0 minutes and 18.0 minutes, respectively, for Patient 1 and 9.1 minutes and 12.1 minutes for Patient 2 (). As expected from the gating window of ± 3 mm in the LR and AP directions and ± 4 mm in the CC direction the distributions of geometrical errors in the gated treatments were within these limits (). Without gating and intrafraction couch corrections the error distributions would be markedly broader due to substantial respiratory induced motion for both patients, and Patient 1 would have considerable mean errors caused by the baseline drift (, ). Patient 2 would not have larger mean errors without gating than with gating due to the relatively stable baseline position (, ).
Figure 2. Distribution of geometrical errors for Patient 1 and 2 during beam-on at fraction 1 and accumulated for all three fractions in the actual treatments with respiratory gating and in simulated treatments without gating. The numbers indicate the mean (upper number) and the standard deviation (lower) of the geometrical error in the left-right (LR), cranio-caudal (CC) and anterior-posterior (AP) direction.
The dose reconstruction for fraction 1 and for the entire treatment course showed good agreement between the CTV dose delivered with gating and the planned CTV dose (, ). Without gating the intrafraction motion would markedly degrade the target coverage for Patient 1 with 3.6% reduction in the CTV mean dose and 12.1% reduction in CTV D95 accumulated over the treatment course (, ). For Patient 2, the dosimetric impact of intrafraction motion would be much smaller due to smaller motion and smaller baseline drift.
Figure 3. Left: Planned dose distributions in a coronal plane in the center of the CTV (red contour) and PTV (blue), and reconstructed dose distributions in the gated treatments and in the simulated non-gated treatments accumulated over fractions 1–3 for Patients 1 and 2. Dose levels > 95% are shown. Right: Corresponding dose-volume histograms for the CTV.
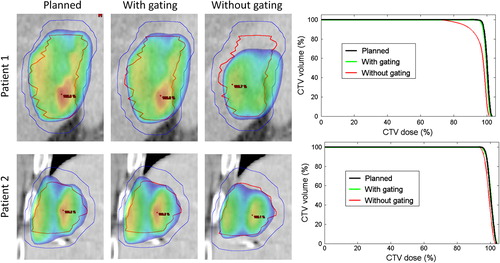
Table II. Difference between delivered and planned CTV dose at the gated treatments and at simulated treatments without gating for both patients (Pt) at fraction 1 (Fx 1) and accumulated over all three fractions (Fx 1–3).
Discussion
Liver SBRT was delivered to two patients with respiratory gating based on internal real-time localization of implanted electromagnetic transponders. Motion analysis and dose reconstruction showed that the gating and the intra-fraction couch corrections largely improved the CTV dose coverage for Patient 1, while the dosimetric gain of the gating was smaller for Patient 2 who had less respiratory motion and baseline drift. The electromagnetic transponder system provided real-time internal motion monitoring on a conventional linear accelerator without the need for ionizing radiation or image processing known from x-ray localization. For both patients, the treatment delivery was efficient with mean duty cycles of approximately 55% and considerably less time spent in the treatment room at fractions 2 and 3 compared to the first fraction (). At fractions 2–3, the mean time between CBCT scanning and treatment start was slightly shorter for Patient 1 (5.5 minutes) than for Patient 2 (8 minutes), for whom the couch was moved laterally to its center position during the scan. However, the treatment delivery time (from first to last field) was longer for Patient 1 (12.9 minutes vs. 8.8 minutes) due to non-coplanar fields and more inter-field couch adjustments. While this first report demonstrates the clinical feasibility and potential accuracy improvements of Calypso-guided respiratory gating for liver SBRT, inclusion of more patients is obviously required for a thorough and statistically sound investigation of the gating technique and its potential benefits.
A gradual systematic intrafraction tumor drift in the cranial direction was observed at all fractions for both patients although this drift was considerably larger for Patient 1 for whom it would have resulted in large CTV dose degradation without the gating and intrafraction couch corrections . A similar systematic drift of the liver has previously been observed in magnetic resonance imaging (MRI) [Citation26].
It should be noted that CTV dose reductions are to be expected in case of substantial tumor motion in the simulated non-gated treatments since the PTV dose gradually decreased from 95% to 67% outside the CTV. The centrally peaked PTV dose prescription allows for a higher CTV dose than obtainable with a uniform isotoxic PTV dose. The better alignment of the CTV with the high dose volume obtained with gating at the same time increases the tumor dose and reduces the healthy tissue dose compared to non-gated treatments. In our standard non-gated liver SBRT treatments, we apply abdominal compression to reduce the respiratory motion. Therefore, the dosimetric impact of the respiratory motion may be less than in the simulated non-gated treatments.
For non-gated treatments in a free-breathing scenario, the treatment planning is typically performed on the mid-ventilation phase of a 4DCT scan [Citation27]. However, the prolonged acquisition time of 4DCT scans complicates the timing of intravenous contrast injection, and 4DCT scans may have severe motion artifacts that compromise the target delineation accuracy [Citation28]. An advantage of the end-exhale gating applied in this study was the possibility to plan the treatment on a contrast enhanced motion artifact free exhale breath-hold CT scan, where the target and Calypso transponders could be delineated directly without any need for image registration and structure propagation between CT volumes. Using the exhale CT scan with 2 mm slice thickness we roughly estimate that the center-of-mass position of each transponder could be identified within an uncertainty of 0.5 mm in the axial plane and 1 mm in the CC direction, We still acquire a 4DCT scan as a part of our Calypso gating protocol in order to verify patient compliance and reliability of the end-exhale breath-hold CT in the free-breathing treatment scenario.
Implanted gold fiducial markers are often applied in image-guided liver SBRT [Citation29] and may be used for intra-treatment motion monitoring by the standard imagers of a conventional linear accelerator [Citation10,Citation27]. Unlike gold markers, the Calypso transponders generate MR artifacts that reduce the potential utilization of post-treatment MRI, but not CT, in follow-up of the patients. The Calypso transponder has a diameter of 1.8 mm and requires a 14G (2.1 mm outer diameter) needle for implantation. In comparison, our conventional gold fiducial marker of 1.0 mm diameter only needs an 18G (1.2 mm outer diameter) needle for implantation. The complication risk increases with increasing diameter of the needle used to perforate the liver. Thus, implantation of Calypso transponders has an increased risk compared to gold fiducials. Complications, mainly bleeding and infection, can usually be managed non-operatively. The risk for complication is considered to increase by two-fold for the Calypso transponders compared to gold fiducials [Citation30]. Experience from other types of needle-based procedures in the liver, such as core biopsies, shows that the procedure results in minor pain in approximately 30% of the patients and severe pain in less than 3%. The frequency of moderate to severe bleeding is less than 0.5% [Citation31].
However, the Calypso transponders have the advantage of 3D localization without use of ionizing radiation. None of the marker-based localization methods provide the actual tumor position as the markers are typically not implanted directly in the tumor in order to avoid seeding of tumor cells [Citation29]. Direct tumor monitoring requires imaging with soft tissue contrast, e.g. by ultrasound [Citation32] or MRI [Citation16,Citation33,Citation34], which is currently not commercially available for gated treatments at a conventional linear accelerator.
The applied dose construction method neglects internal tissue deformation and models internal target motion as rigid motion of the entire patient [Citation24]. The mean dose error in the high dose volume by this simplification was estimated to be around 1% when averaged over the entire breathing cycle for a liver SBRT patient with large breathing induced displacements of nearly 3 cm [Citation10]. Patient 1 in the current study had similarly large breathing motion () so errors of the same magnitude may be expected for the reconstructed dose distributions for this patient in . A possible CTV shift relative to the transponder positions is unknown and not accounted for in the dose reconstruction.
Although the improvements in geometric accuracy with the Calypso-guided respiratory gating may allow margin reductions, we used our standard CTV-PTV margins for non-gated treatments in this study. Experience with more patients and more thorough data evaluation (e.g. of migration assessed by the individual transponder positions) will form the basis for possible treatment improvements, such as margin reductions and real-time motion adaptation by multi-leaf collimator (MLC) tracking, rather than by gating. Calypso-guided MLC tracking has been clinically demonstrated for prostate treatments on a conventional linear accelerator [Citation35] and could prove very valuable for liver SBRT due to the large target motion.
In summary, respiratory gating based on the internal motion of electromagnetic transponders was reported for the first time for liver SBRT. For one of two patients, the improved accuracy of gated compared to standard non-gated treatment delivery resulted in a largely improved CTV dose coverage.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported The Danish Cancer Society, CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology, and Varian Medical Systems, Inc., Palo Alto, CA.
References
- Høyer M, Muren LP. Stereotactic body radiation therapy – a discipline with Nordic origin and profile. Acta Oncol 2012;51:564–7.
- Høyer M, Swaminath A, Bydder S, Lock M, Méndez Romero A, Kavanagh B, et al. Radiotherapy for liver metastases: A review of evidence. Int J Radiat Oncol Biol Phys 2012; 82:1047–57.
- Fode MM, Høyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol 2015;114:155–60.
- Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croisé-Laurent V, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol 2015;115:211–6.
- Aitken KL, Tait DM, Nutting CM, Khabra K, Hawkins MA. Risk-adapted strategy partial liver irradiation for the treatment of large volume metastatic liver disease. Acta Oncol 2014;53:702–6.
- Dahele M, Hatton M, Slotman B, Guckenberger M. Stereotactic body radiotherapy: A survey of contemporary practice in six selected European countries. Acta Oncol Epub2015 Mar 3.
- Mendez Romero A, Zinkstok RT, Wunderink W, van Os RM, Joosten H, Seppenwoolde Y, et al. Stereotactic body radiation therapy for liver tumors: Impact of daily setup corrections and day-to-day anatomic variations on dose in target and organs at risk. Int J Radiat Oncol Biol Phys 2009;75:1201–8.
- Park JC, Park SH, Kim JH, Yoon SM, Song SY, Liu Z, et al. Liver motion during cone beam computed tomography guided stereotactic body radiation therapy. Med Phys 2012;39:6431–42.
- Worm ES, H yer M, Fledelius W, Hansen AT, Poulsen PR. Variations in magnitude and directionality of respiratory target motion throughout full treatment courses of stereotactic body radiotherapy for tumors in the liver. Acta Oncol 2013;52:1437–44.
- Poulsen PR, Worm ES, Petersen JBB, Grau C, Fledelius W, Høyer M. Kilovoltage intrafraction motion monitoring and target dose reconstruction for stereotactic volumetric modulated arc therapy of tumors in the liver. Radiother Oncol 2014;111:424–30.
- Gabrys D, Kulik R, Trela K, Ślosarek K. Dosimetric comparison of liver tumour radiotherapy in all respiratory phases and in one phase using 4DCT. Radiother Oncol 2011;100:360–4.
- Xi M, Zhang L, Liu MZ, Deng XW, Huang XY, Liu H. Dosimetric analysis of respiratory-gated radiotherapy for hepatocellular carcinoma. Med Dosim 2011;36:213–8.
- Berbeco RI, Neicu T, Rietzel E, Chen GTY, Jiang SB. A technique for respiratory-gated radiotherapy treatment verification with an EPID in cine mode. Phys Med Biol 2005;50:3669–79.
- Li R, Mok E, Chang DT, Daly M, Loo BW, Jr., Diehn M, et al. Intrafraction verification of gated RapidArc by using beam-level kilovoltage x-ray images. Int J Radiat Oncol Biol Phys 2012;83:e709–15.
- Law AL, Ng WT, Lee MCH, Chan ATS, Fung KH, Li F, et al. Treatment of primary liver cancer using highly- conformal radiotherapy with kV-image guidance and respiratory control. Radiother Oncol 2012;102:56–61.
- Paganelli C, Seregni M, Fattori G, Summers P, Bellomi M, Baroni G, et al. Magnetic resonance imaging-guided versus surrogate-based motion tracking in liver radiation therapy: A prospective comparative study. Int J Radiat Oncol Biol Phys 2015;91:840–8.
- Gierga DP, Brewer J, Sharp GC, Betke M, Willett CG, Chen GTY. The correlation between internal and external markers for abdominal tumors: Implications for respiratory gating. Int J Radiat Oncol Biol Phys 2005; 61:1551–8.
- Kitamura K, Shirato H, Seppenwoolde Y, Shimizu T, Kodama Y, Endo H, et al. Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys 2003;56:221–8.
- Kupelian P, Willoughby T, Mahadevan A, Djemil T, Weinstein G, Jani S, et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:1088–98.
- James J, Cetnar A, Nguyen VN, Wang B. Commissioning of radiofrequency tracking for gated SBRT of the liver using novel motion system. Med Phys 2015;42:3582
- Smith RL, Sawant A, Santanam L, Venkat RB, Newell LJ, Cho B, et al. Integration of real-time internal electromagnetic position monitoring coupled with dynamic multileaf collimator tracking: An intensity-modulated radiation therapy feasibility study. Int J Radiat Oncol Biol Phys 2009; 74:868–75.
- Lax I. Target dose versus extratarget dose in stereotactic radiosurgery. Acta Oncol 1993;32:453–7.
- Lax I, Panettieri V, Wennberg B, Duch MA, Näslund I, Baumann P, et al. Dose distributions in SBRT of lung tumors: Comparison between two different treatment planning algorithms and Monte-Carlo simulation including breathing motions. Acta Oncol 2006;45:978–88.
- Poulsen PR, Schmidt ML, Keall P, Worm ES, Fledelius W, Hofffmann L. A method of dose reconstruction for moving targets compatible with dynamic treatments. Med Phys 2012;39:6237–46.
- Ravkilde T, Keall PJ, Grau C, H yer M, Poulsen PR. Time-resolved dose reconstruction by motion encoding of volumetric modulated arc therapy fields delivered with and without dynamic multi-leaf collimator tracking. Acta Oncol 2013;52:1497–503.
- von Siebenthal M, Szekely G, Lomax AJ, Cattin PC. Systematic errors in respiratory gating due to intrafraction deformations of the liver. Med Phys 2007;34:3620–9.
- Worm ES, H yer M, Fledelius W, Poulsen PR. Three-dimensional, time-resolved, intrafraction motion monitoring throughout stereotactic liver radiation therapy on a conventional linear accelerator. Int J Radiat Oncol Biol Phys 2013;86:190–7.
- Yamamoto T, Langner U, Loo BW, Jr., Shen J, Keall PJ. Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int J Radiat Oncol Biol Phys 2008;72:1250–8.
- Seppenwoolde Y, Wunderink W, Wunderink-van Veen SR, Storchi P, Romero AM, Heijmen BJM. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker-tumour distance. Phys Med Biol 2011;56:5445–68.
- Mueller M, Kratzer W, Oeztuerk S, Wilhelm M, Mason RA, Mao R, et al. Percutaneous ultrasonographically guided liver punctures: An analysis of 1961 patients over a period of ten years. BMC Gastroenterol 2012;12:173.
- Howlett DC, Drinkwater KJ, Lawrence D, Barter S, Nicholson T. Findings of the UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor and major complications and procedure-related mortality. Radiology 2013;266:226–35.
- Bell MA, Byram BC, Harris EJ, Evans PM, Bamber JC. In vivo liver tracking with a high volume rate 4D ultrasound scanner and a 2D matrix array probe. Phys Med Biol 2012;57:1359–74.
- Wooten HO, Rodriguez V, Green O, Kashani R, Santanam L, Tanderup K, et al. Benchmark IMRT evaluation of a Co-60 MRI-guided radiation therapy system. Radiother Oncol 2015;114:402–5.
- Heerkens HD, van Vulpen M, van den Berg CA, Tijssen RH, Crijns SP, Molenaar IQ, et al. MRI-based tumor motion characterization and gating schemes for radiation therapy of pancreatic cancer. Radiother Oncol 2014; 111:252–7.
- Keall PJ, Colvill E, O’Brien R, Ng JA, Poulsen PR, Eade T, et al. The first clinical implementation of electromagnetic transponder-guided MLC tracking. Med Phys 2014; 41:020702.
