ABSTRACT
Background. To evaluate efficacy and toxicity of radio-chemotherapy (RCT) and MR-guided pulsed-dose-rate (PDR) adaptive brachytherapy (IGABT) for locally advanced cervical cancer (LACC).
Material and methods. Between 2007 and 2014 85 patients with FIGO stage 1B1 N+ or ≥ 1B2 cervical cancer were treated with RCT+ IGABT. The treatment consisted of a pelvic± paraaortic external beam radiotherapy (EBRT) (45–50.4 Gy ± 10 Gy boost to primary tumor and/or to pathologic lymph nodes) with concurrent cisplatin followed by 25–35 Gy of PDR IGABT in 30–50 pulses. The ratio of 3D-CFRT/IMRT was 61/24 patients. Dose-volume parameters of high-risk clinical target volume (HR-CTV), intermediate-risk clinical target volume (IR-CTV) and D2cm3 organs at risk (OARs) were reported. Local control (LC), cancer-specific survival (CCS) and overall survival (OS) were analyzed actuarially and morbidity crude rates were scored using CTCAEv4.0.
Results. Mean follow-up was 36 months (range 6–94). The mean D90 and D98 for HR-CTV was 84.4 ± 9 Gy and 77 ± 8.1 Gy, while for IR-CTV was 69.1 ± 4.3 Gy and 64.8 ± 4.3 Gy, respectively. The mean D2cm3 for OARs was the following: bladder: 77.3 ± 10.5 Gy, rectum: 65 ± 6.8 Gy, sigmoid: 63 ± 7.9 Gy and intestine: 64.0 ± 9.1 Gy. Three year LC, CSS and OS were: 94%, 85% and 81%. The three-year regional- and distant control rates were 95% and 74%. Node negative patients had significantly higher three-year CSS (100 vs. 72%, p = 0.016) and OS (92 vs. 72%, p = 0.001) compared to node positive ones. Three-year actuarial late Grade ≥ 3 morbidity was the following: GI: 8%, GU: 5%, Vaginal: 8%. The frequency of Grade ≥ 3 hematological toxicities including anemia/leukopenia/neutropenia/thrombocytopenia were 8.6%/34.7%/24.3%/24.3%, respectively.
Conclusion. This large mono-institutional experience builds up further evidences that IGABT in conjunction with RCT should be the standard of care for patients suffering LACC.
Concurrent radio-chemotherapy (RCT) followed by image-guided adaptive brachytherapy (IGABT) is the treatment of choice for locally advanced cervical cancer (LACC) [Citation1–4]. Randomized trials have found that RCT improves local, distant tumor control and survival compared with RT alone [Citation1]. The last two decades brought a major evolution of brachytherapy technology. Introduction of magnetic resonance imaging (MRI) [Citation5,Citation6], multifunctional – combined intracavitary and interstitial (IC/IS) – applicators [Citation7,Citation8] and magnetic resonance imaging (MRI)-based planning [Citation9] have significantly improved the accuracy of BT. These kind of procedures were rapidly gaining popularity in the BT community resulting in a series of GEC-ESTRO (Group Européen de Curiethérapie and the European Society for Radiotherapy and Oncology) recommendation guidelines for target volume definition, dose planning reporting, applicator reconstruction and MRI [Citation2,Citation3,Citation10,Citation11]. Several leading centers have already published their first results with IGABT [Citation4,Citation12–16]. The clinical benefit of IGABT is meaningful yielding three-year local control (LC), cancer-specific survival (CSS) and overall survival (OS) rates of 91–95%, 74–87% and 65–79% with reduced rate of treatment related morbidity [Citation4,Citation8, Citation12–15,Citation17,Citation18]. Emerging evidences are awaited from a large, prospective, multicenter study [International Study on Magnetic Resonance Imaging-guided Brachytherapy in Locally Advanced Cervical Cancer (EMBRACE) (http://www.embracestudy.dk)] and a retrospective analysis (RETROEMBRACE) conducted by the EMBRACE participants [Citation19].
At University Hospital of Liège MRI-based pulsed-dose-rate (PDR) BT planning was introduced in 2007 and showed continuous progress during the last seven years. In this paper we aimed to report the clinical outcome of the first 85 patients treated with RCT and IGABT supporting further evidence for this treatment approach.
Material and methods
Patients
Between January 2007 and November 2014 93 biopsy proven LACC patients were treated with combined external beam radiotherapy (EBRT) and single agent weekly cisplatin (40 mg/m2) followed by MR-IGABT. Eight patients were excluded from the analysis due to neoadjuvant chemotherapy (2), missing follow-up data (4) and incomplete BT (2). Eventually, 85 patients were included in this retrospective analysis. Staging consisted of gynecological examination according to Fédération Internationale de Gynécologie et d’Obstétrique (FIGO), whole body 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) and abdominal-pelvic MRI in all patients. Cystoscopy or rectoscopy was added if organ infiltration was suspected. Laparoscopic elective paraaortic (PAO) lymph node assessment was performed in each clinically node negative, medically fit patient to exclude microscopic disease and eventually determine the superior limit of the radiation field. In case of enlarged metastatic pelvic or PAO lymph nodes (short axis ≥ 2 cm) a lymph node debulking was performed ().
Table I. Patient and tumor characteristics.
Treatment
Treatment characteristics are presented in . From January 2007 to April 2013 all patients were treated with three-dimensional conformal radiotherapy (3D-CFRT) by four-field box technique. The elective pelvic dose was 45 Gy in 25 fractions in the first two cases then systematically 50.4 Gy in 28 fractions (fx). Additionally a sequential boost of 10 Gy in 5 fx was given to the primary tumor and/or to the pathological lymph nodes in case of FIGO stage ≥ IIBdistal and/or nodal metastasis by three-field technique. From April 2013 3D-CFRT was replaced by IMRT and VMAT maintaining a pelvic dose of 50.4 Gy in 28 fx. The sequential boost to the pathologic nodes was also replaced by simultaneously integrated boost (SIB) of 58 Gy in 28 fx. The primary tumor boost was abandoned as well and replaced by IGABT. PAO RT with 45 Gy in 25 fx were prescribed in case of pathological common iliac or PAO nodes either proven histologically after surgical staging or identified at PET-CT or MRI.
Table II. Treatment characteristics.
MR-guided PDR BT was performed in all cases. IC-BT was delivered using initially a titanium tandem-ovoid applicator (Varian Medical Systems, Gammamed) which was replaced by a plastic tandem-ring applicator (T/R; GammaMed; Varian Medical Systems, Palo Alto, CA) from 2012. In 2013 a combined IC/IS technique has been introduced using a home-made plastic cap with steering holes for plastic IS needle implantation. The BT schedule consisted of mainly one application. Two applications were only performed for IC/IS interventions. In such cases both procedure were realized on the same day. At first a T/R applicator with needle cap was inserted followed by MRI which was used for planning the IS needle trajectory. The second intervention served for needle placement followed by a second MRI/CT for definitive planning purposes. The applied MRI sequences have been modified with time. The initially used 2D paraxial, parasagittal- and para-coronal 1.5 Tesla images were replaced by 3 Tesla 3D sequences. The number of PDR pulses was determined in function of the delivered EBRT dose to the primary tumor: 60 Gy + 25 Gy in 42 pulses, 45–50.4 Gy + 30–35 Gy in 50 pulses.
Dose calculation and reporting were based on the total biologically equivalent dose in 2-Gy fractions (EQD2) of EBRT+ BT using the linear quadratic model with α/β = 10 Gy for tumor and α/β = 3 Gy for OARs, and a repair half time of 1.5 hours. Initially the planning aim for D90 high-risk clinical target volume (HR-CTV) was ≥ 80 Gy for IB2 and proximal IIB disease, while 85 Gy for more advanced disease stages. From May 2012 the planning aim has been harmonized to 85 Gy for every patient. The planning aim for the OARs did not change with time: D2cm3 for bladder ≤ 90 Gy and ≤ 75 Gy for the rectum, sigmoid and bowel.
Target and organ at risk (OAR) delineation [gross tumor volume (GTV), HR-CTV, intermediate risk clinical target volume (IR-CTV), bladder, rectum, sigmoid colon and bowel] and dose reporting was performed according to the GEC-ESTRO recommendations [Citation2,Citation3,Citation10].
Outcome
Complete clinical remission was defined as no evidence of disease three months after completion of treatment, evaluated by clinical examination, MRI and PET-CT. Three-year actuarial rates for LC, OS, CSS and progression-free survival (PFS) were calculated. OS/CSS were defined as the period from the date of start of EBRT to date of any death/death by cervical cancer. PFS was subdivided in PFSlocal, PFSregional, PFSpelvic, PFSdistant and PFSoverall as the interval from start of treatment to local, regional, loco-regional, distant or any failure. PFSlocal/regional/pelvic included persistent diseases as well. All follow-up were calculated from date of start of treatment to last visit or death of the patient.
Morbidity
Both acute hematological (HT)/renal toxicity and late morbidity was scored using the Common Terminology Criteria for Adverse Events (CTCAE 4.0). For late gastrointestinal (GI), genito-urinary (GU) toxicity both crude and actuarial rates were reported. As the side effects were not formally and prospectively scored in the past, only the major, Grade 3–4 morbidities were presented to keep reliable and objective data reporting.
Follow-up and statistics
Patients were followed with gynecological examination every three months the first year, every six months in year 2–3 and every 12 months in year 4–5. All patients were scanned with MRI and PET-CT at three-month follow-up and again at 12-month follow-up. MRI and PET-CT were also performed if a recurrence was clinically suspected.
Statistical analysis was performed with SAS version 9.2 (SAS Institute, Cary, NC, USA) statistical package. Descriptive statistics were used to characterize the clinical variables. OS, CSS, PFS were described with Kaplan-Meier method. Outcome parameters and late morbidity were calculated at three years. The log-rank test was used to compare survival curves. A p-value of < 0.05 was considered significant.
Results
Patient-, tumor- and treatment characteristics
Data are presented in and . Median age was 50 years (26–78). The dominant FIGO stage was IIB (40%), while more than 50% of the patients had an initial tumor size ≥ 5 cm. The vast majority of the patients (86%) had squamous cell cancer. Forty-five patients (53%) had either clinically or pathologically confirmed lymph node metastasis. Sixty-six patients (78%) had laparoscopic lymph node dissection including 59 cases with PAO lymph node assessment (70%). Thirty-nine patients (46%) were active smoker at the time of the treatment, while 36 patients (42%) had documented previous abdominal surgery.
Sixty-one patients (71%) were treated with 3D-CFRT, while in the remaining cases (n = 24) IMRT/VMAT was used. Twenty-one patients received PAO irradiation (25%) and two patients (2%) had groin irradiation. Thirty-six patients (42%) received nodal boost (Sequential: 28, SIB: 8). Primary tumor boost of 60 Gy was delivered in 18 patients (21%). IC-BT was performed in 75 patients (88%), while IC/IS technique was applied in 10 cases (12%). The median overall treatment time was 49 (40–81) days and all patients received at least one cycle of concomitant chemotherapy.
Dose-volume parameters
Dose-volume parameters are presented in . The mean HR-CTV volume was 38.07 ± 27.62 cm3. The mean D90 and D98 for HR-CTV was 84.4 ± 9 Gy and 77 ± 8.1 Gy, while for IR-CTV was 69.1 ± 4.3 Gy and 64.8 ± 4.3 Gy, respectively. The mean D2cm3 for OARs was the following: bladder: 77.3 ± 10.5 Gy, rectum: 65 ± 6.8 Gy, sigmoid: 63 ± 7.9 Gy and intestine: 64.0 ± 9.1 Gy.
Table III. Dose-volume parameters in EQD2.
Disease control
The mean follow-up was 36 (range 6–94) months. Clinical outcomes are summarized in and and .
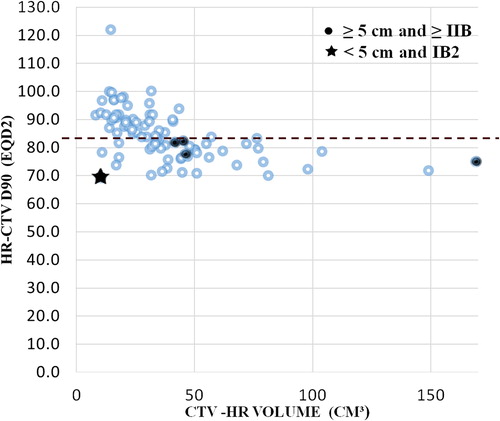
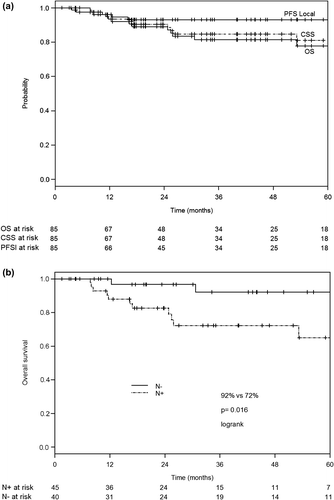
Table IV. Disease control with IGABT.
Failures occurred in 18 patients, including five local-, three nodal- and 18 distant recurrences.
Complete remission was achieved in 83/85 patients (97.6%). Two patients had persistent disease at completion of treatment and eventually progressive locale disease. Both patients developed distant metastasis within three months after local progression. Three patients had a central local recurrence after initial complete clinical remission, including one patient with exclusive local failure and two patients with simultaneous distant metastasis with or without nodal failure. The only patient with exclusive pelvic recurrence was salvaged by neoadjuvant chemotherapy followed by pelvic exenteration. The patient eventually failed both locally and at distant sites four months after the radical surgery. Finally three of five patients died from the direct consequences of the local failure. The three-year PFSlocal was 94%. As shown in , four of five patients with local failure had an initial tumor size ≥ 5 cm and a HR-CTV volume ≥ 40 cm3. No recurrence occurred when ≥ 85 Gy HR-CTV D90 was delivered.
Three patients developed pelvic regional failure, including one patient with persistent disease after initial treatment. Two of three patients died within six months after the failure was diagnosed. Each patient had simultaneous PAO lymph node metastasis including one patient with multiple visceral metastases and one patient with local failure. Two patients had a positive nodal status at diagnosis and received an EBRT nodal boost. Thus, the three-year PFSregional was 95%. Altogether eight pelvic failures (either local or regional) occurred in seven patients resulting in a three-year PFSpelvic of 90%.
Eighteen patients developed distant metastasis including three patients with PAO failure only, six patients with other distant organ failure and nine patients with both. The main sites of metastasis were PAO region, lung, bone, mediastinal lymph nodes and peritoneum. Two of the three isolated PAO recurrences were salvaged by RCT while the remaining one had a persistent disease after EBRT with co-existing resistant pelvic lymph nodes. The three-year PFSdistant was 74%. Node positive patients had higher risk to develop distant failure as compared to the node negative ones (63% vs. 85%, p = 0.06).
Seventy-one patients were alive at last follow-up (83.5%), 12 patients died due to cancer and only two patients because of other causes. The three-year CSS and OS were 85% and 81%, respectively (). Node negative patients had significantly better three-year OS and CSS survival rates compared to node positive ones (92% vs. 72%, p = 0.016; 100% vs. 72%, p = 0.001, ).
Toxicity
The median number of completed chemotherapy cycles was six (range 1–8). More than 80% of patients received five cycles. The main reason of uncompleted therapy was HT. The most frequent Grade ≥ 3 HT in decreasing order was leukopenia (37.3%), neutropenia (24.3%), thrombocytopenia (24.3%) and anemia (8.6%) (Supplementary Table I, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1062542). Severe renal function impairment was rare, affecting 3% of the patients.
In regard to late non-HT morbidity altogether 20 major events were observed in 12 patients. Details are presented in . The three-year actuarial rate for Grade 3–4 morbidity was 5% for urinary-, 8% for GI- and 8% for vaginal toxicity. Three patients presented combined Grade ≥ 3 side effects and almost all toxicities (10/12) occurred within the first three years.
Table V. Severe late adverse events (AEs) with IGABT.
Five patients developed eight major GI events, including four fistulas (one colon-uterus, two rectum-vagina, one small bowel-vagina), three bowel obstructions and one proctitis. Three patients presented more than one events. Three of four patients with fistula developed finally GI obstructive symptoms and all patients needed elective surgery and colostomy. The rectal D2cm3 values respected the planning objectives: rectum: 66.7 ± 1.3 Gy, sigmoid: 64.6 ± 7.8 Gy and bowel 62.7 ± 8.9 Gy. All four patients with diabetes developed severe late GI morbidity.
Seven major urinary events were recorded for five patients, including two hemorrhagic cystitis, one ureter stenosis, two fistulas (one ureter-vagina, one bladder-vagina) and two incontinences. One patient with FIGO stage III/B tumor developed after complete remission a Grade 2 cervical necrosis with ureter stenosis and co-existent ureter-vaginal fistula resulting in a urinary incontinence. She had a ureter resection and re-implantation. Two patients suffered from hemorrhagic cystitis, needed transfusion and endoscopic cauterization. The average bladder D2cm3 was 76.7 ± 9 Gy, remaining in each case within the planning aims.
No correlation was seen between D2cm3 of the OARs and the development of morbidity. The ≥ Grade 3 vaginal side effects were stenosis affecting five patients in the population.
Discussion
Concomitant chemo-irradiation is the standard of care for patients suffering advanced cervical cancer. Recently, significant improvements in the LC rate, OS and toxicity profile have been achieved following the introduction of IGABT. We aim to report the clinical outcome of 85 patients treated with concurrent RCT and PDR IGABT at the CHU of Liège over a seven-year period. Overall, three-year PFSlocal, CSS and OS of 94%, 85% and 81% were achieved, respectively. As shown in these results are comparable to those published in the recent international literature [Citation1–6] confirming the added value of the IGABT concept. Indeed, one prospective and four retrospective studies have confirmed that IGABT significantly improves pelvic/LC with reduced toxicity as compared to standard BT. In the Vienna, Aarhus, Utrecht and Leiden series the three-year local/pelvic control improved from 64–76% to 85.5–93.3%, while the three-year OS increased from 51–63% to 65–85.6% if IGABT was used. These results suggest that improved pelvic control rates achieved by IGABT could be responsible for better survival.
The most important prognostic factors on LC are D90 HR-CTV and volume of HR-CTV [Citation4,Citation13–16,Citation20,Citation21]. A dose-response relationship was first demonstrated by Dimopoulos et al. [Citation21] showing that three-year LC rates ≥ 95% can be achieved when the D90 HR-CTV is higher than 87 Gy. The authors thus recommended an EQD2 dose ≥ 85 Gy. Preliminary results of the multicenter RETROEMBRACE study have confirmed this dose-response relationship on 592 patients [Citation19]. Achieving this dose level is however difficult without adapting and optimizing the BT technique. Pötter et al. [Citation4] reported LC rates of 86% for stage IIIB tumors and 92% for tumors > 5 cm using combined IC/IS technique in more than 40% of the cases. From 2007 to May 2012 we treated large (≥ IIB distal) tumors with 60 Gy EBRT followed by an adapted, reduced dose of IC-BT. As a consequence of the above mentioned results we re-optimized our MRI acquisitions, fixed our planning aim of HR-CTV to 85 Gy and as a latest step introduced combined IC/IS technique. In our analysis HR-CTV volume also seemed to be a contributing factor on local tumor control. Decreased D90 HR-CTV and larger tumors had a trend toward reduced LC as well. There was only one failure with less than 40 cm3 of HR-CTV volume and no local failure/progression occurred above 85 Gy (). Other factors, such as FIGO stage, histology, OTT [Citation4,Citation13,Citation15,Citation16,Citation20] were also reported to predict LC, however our relatively small number of patients with only five events does not allow reliable univariate/multivariate or subgroup analysis on LC. It should be noted that our patient population slightly differ from the published ones presenting relatively fewer locally advanced stage tumors () might affect the LC positively.
In the first period of the analysis the ratio of fulfilled planning aims remained below 50% (Supplementary Table II, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1062542). It can be partially explained by the fact that 60 Gy EBRT did not solve the HR-CTV size coverage problem. Moreover, this increased background dose further limited the optimization window between OARs and HR-CTV, which was basically already compromised with IC-BT with large HR-CTV volumes. By using IS/IC technique both of these difficulties could be overcome as it is showed by Mohamed et al. [Citation22]. The implementation of this technique has been a stepwise project at CHU de Liège and currently almost 30% of the patients are treated with needles.
Nodal failure was rare in our series, however once it occurred; the outcome of these patients was very poor leading to metastatic death within six months after the treatment. The presence of lymph node metastasis was significant prognostic factor for OS. Node positive patients had higher risk for developing distant metastasis, overall progression and they had significantly worse three-year CSS and OS () as compared to node negative ones, as also reported by Nomden et al. [Citation14].
The dominant pattern of failure was distant metastasis affecting 21.2% of the patients which are in line with the literature () [Citation4,Citation13–15]. Patient with locally advanced diseases and/or nodal metastasis are at higher risk for developing distant failure. Intensified systemic treatments, such as chemotherapy either before or after RCT aiming at reducing the rate of distant relapses are currently tested in prospective randomized trials (OUTBACK, http://clinicaltrials.gov/show/NCT01414608, INTERLACE, http://www.cancerresearchuk.org/cancer-help/trials/a-trial-of-chemotherapybefore-chemoradiation-for-cervical-cancer-interlace).
One of the most common acute side effects during RCT is hematopoietic. Severe (≥ Grade 3) HT side effects occur in up to 30% of patients [Citation1]. In our experience, we confirmed a 24–35% rate of for leukopenia, neutropenia and thrombocytopenia. It should be also mentioned that an average of five radio-sensitizing chemotherapy cycles were delivered. Prevention of radiation-induced HT may impact on the global tolerance to RCT and let the patient capable to tolerate more intensified adjuvant systemic treatment. Moreover, by the increasing use of IS needles a maintained HT status becomes even more important. Bone marrow sparing strategies are currently investigated in a prospective randomized trial (INTERTECC http://clinicaltrials.gov/show/NCT01554397).
Despite respecting dose constraints to OARs the three-year actuarial rate for late Grade 3–4 morbidity was 5% for urinary-, 8% for GI- and 8% for vaginal toxicity, exceeding the reported numbers in the literature (1–4%, 2–3%,1–4%) [Citation4,Citation12–16]. GI morbidity compares to the Utrecht and Leiden data but remains inferior to those reported by Vienna and Aarhus groups. Urinary toxicity is comparable with the Vienna results, but definitely higher than in the other series (). Again, direct comparisons with the published series should be handled cautiously as several patient-, tumor- and treatment-related factors may contribute to morbidity. Each patient file with such adverse events was re-examined. Applicator reconstruction, target and OARs contouring were found to be adequate in each case. Neither hot spots in the cervix area nor adjacent lymph node boost were noted on the EBRT plans. All but one patient were treated by IMRT. Nomden et al. [Citation23] recently published significant uncertainties in rectum/sigmoid mean doses during 31 hours PDR treatments evaluated by repetitive MR scans. The mean rectal dose was found to increase significantly over time. Mazeron et al. [Citation24] has also evaluated the intrafractional movements during long course PDR treatments by repetitive scans on Days 1, 2, and 3. They reported an increase of 6% of the planned D2cm3 in EQD2 of the rectum affected most patients resulting in an unacceptably high delivered D2cm3 in 10.5% of the patients. Important random variations were also observed. Our treatment schedule consists of a 50-hour single application PDR treatment without use of an intrarectal probe. Based on these observations uncertainties related to intrafractional dosimetric variations could contribute to the late side effects observed in our series.
From a toxicity perspective it is recognized that the risk of late GI/GU side effects increases with a history of abdominal surgery, pelvic inflammatory disease, hypertension, diabetes mellitus and smoking [Citation25,Citation26]. Interestingly, each diabetic patient in our population developed severe GI side effects. All patients with GI side effects were heavy smokers as well as the patient with ureteric fistula and stenosis.
Finally one cannot exclude the impact of EBRT on late side effects. Late ≥ Grade 2 GI toxicity after definitive RCT is about 20–30%. The majority of the patients (72%) were treated with conventional 3D-CRT. Increasing evidence exist that IMRT could reduce acute Grade 2–3 GI side effects by 20–30% as compared to 3D planning [Citation27]. Limited results are available about the definitive effect of IMRT on late toxicity, however, the preliminary results are promising [Citation27]. Also library-based planning with daily online image guidance is upcoming approach to further decrease PTV margins and ultimately the dose delivery to OARs [Citation28].
In conclusion, our results build up further evidences that IGABT in conjunction with RCT should be the standard of care for patients suffering LACC. Improvement of late toxicity could be awaited from increasing use of IC/IS-BT and the implementation of individualized adaptive IGRT/IMRT. Additionally, this database will be expanded and will contribute to evaluate the added value of adaptive and/or bone marrow sparing RT techniques in the near future.
Supplementary material available online
Supplementary Tables 1–2 available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1062542
ionc_a_1062542_sm1965.docx
Download MS Word (18.5 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: Individual patient data meta-analysis. Cochrane Database Syst Rev 2010;CD008285.
- Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45.
- Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77.
- Pötter R, Georg P, Dimopoulos JC, Grimm M, Berger D, Nesvacil N, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23.
- Schmid MP, Fidarova E, Pötter R, Petric P, Bauer V, Woehs V, et al. Magnetic resonance imaging for assessment of parametrial tumour spread and regression patterns in adaptive cervix cancer radiotherapy. Acta Oncol 2013;52:1384–90.
- van de Schoot AJAJ, de Boer P, Buist MR, Stoker J, Bleeker MCG, Stalpers LJA, et al. Quantification of delineation errors of the gross tumor volume on magnetic resonance imaging in uterine cervical cancer using pathology data and deformation correction. Acta Oncol 2015;54:224–31.
- Nomden CN, de Leeuw AAC, Moerland MA, Roesink JM, Tersteeg RJH, Jürgenliemk-Schulz IM. Clinical use of the Utrecht applicator for combined intracavitary/interstitial brachytherapy treatment in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2012;82:1424–30.
- Fokdal L, Tanderup K, Hokland SB, Røhl L, Pedersen EM, Nielsen SK, et al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol 2013;107:63–8.
- Palmqvist T, Dybdahl Wanderås A, Langeland Marthinsen AB, Sundset M, Langdal I, Danielsen S, et al. Dosimetric evaluation of manually and inversely optimized treatment planning for high dose rate brachytherapy of cervical cancer. Acta Oncol 2014;53:1012–8.
- Hellebust TP, Kirisits C, Berger D, Pérez-Calatayud J, De Brabandere M, De Leeuw A, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: Considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol 2010;96:153–60.
- Dimopoulos JCA, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol 2012;103:113–22.
- Charra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol 2012;103:305–13.
- Lindegaard JC, Fokdal LU, Nielsen SK, Juul-Christensen J, Tanderup K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52:1510–9.
- Nomden CN, de Leeuw AAC, Roesink JM, Tersteeg RJHA, Moerland MA, Witteveen PO, et al. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: A single institution experience. Radiother Oncol 2013;107:69–74.
- Rijkmans EC, Nout RA, Rutten IHHM, Ketelaars M, Neelis KJ, Laman MS, et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol 2014;135:231–8.
- Gill BS, Kim H, Houser CJ, Kelley JL, Sukumvanich P, Edwards RP, et al. MRI-guided high-dose-rate intracavitary brachytherapy for treatment of cervical cancer: The University of Pittsburgh experience. Int J Radiat Oncol Biol Phys 2015;91:540–7.
- Fokdal L, Tanderup K, Nielsen SK, Christensen HK, Røhl L, Pedersen EM, et al. Image and laparoscopic guided interstitial brachytherapy for locally advanced primary or recurrent gynaecological cancer using the adaptive GEC ESTRO target concept. Radiother Oncol 2011;100:473–9.
- Haie-Meder C, Chargari C, Rey A, Dumas I, Morice P, Magné N. DVH parameters and outcome for patients with early-stage cervical cancer treated with preoperative MRI-based low dose rate brachytherapy followed by surgery. Radiother Oncol 2009;93:316–21.
- Tanderup K, Fokdal L, Sturdza AE, Mazeron R, Kirisits C, Lindegaard JC. Dose-response for local control in image guided cervix brachytherapy in the retro EMBRACE study. Radiother Oncol 2013;106:S103–4.
- Mazeron R, Castelnau-Marchand P, Dumas I, Del Campo ER, Kom LK, Martinetti F, et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother Oncol 2015;114:257–63.
- Dimopoulos JCA, Lang S, Kirisits C, Fidarova EF, Berger D, Georg P, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2009;75:56–63.
- Mohamed S, Kallehauge J, Fokdal L, Lindegaard JC, Tanderup K. Parametrial boosting in locally advanced cervical cancer: Combined intracavitary/interstitial brachytherapy vs. intracavitary brachytherapy plus external beam radiotherapy. Brachytherapy 2015;14:23–8.
- Nomden CN, de Leeuw AAC, Roesink JM, Tersteeg RJH, Westerveld H, Jürgenliemk-Schulz IM. Intra-fraction uncertainties of MRI guided brachytherapy in patients with cervical cancer. Radiother Oncol 2014;112:217–20.
- Mazeron R, Champoudry J, Gilmore J, Dumas I, Goulart J, Oberlander A-S, et al. Intrafractional organs movement in three-dimensional image-guided adaptive pulsed-dose-rate cervical cancer brachytherapy: Assessment and dosimetric impact. Brachytherapy 2015;14:260–6.
- Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician 2010;82:381–8, 394. [cited 2015 May 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20704169.
- Levitchi M, Charra-Brunaud C, Quetin P, Haie-Meder C, Kerr C, Castelain B, et al. Impact of dosimetric and clinical parameters on clinical side effects in cervix cancer patients treated with 3D pulse-dose-rate intracavitary brachytherapy. Radiother Oncol 2012;103:314–21.
- Mazeron R, Dumas I, El Khouri C, Lévy A, Attar M, Haie-Meder C. [Intensity-modulated radiotherapy in cervical cancer: towards a new standard?]. Cancer Radiother 2014;18:154–60; quiz 162, 164.
- Ahmad R, Bondar L, Voet P, Mens J.-W, Quint S, Dhawtal G, et al. A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: A dosimetric evaluation. Acta Oncol 2013;52:1430–6.
