ABSTRACT
Background. Gastrointestinal (GI) morbidity after radiotherapy (RT) for prostate cancer is typically addressed by studying specific single symptoms. The aim of this study was to explore the interplay between domains of patient- reported outcomes (PROs) on GI morbidity, and to what extent these are explained by RT dose to the GI tract.
Material and methods. The study included men from two Scandinavian studies (N = 211/277) who had undergone primary external beam radiotherapy (EBRT) for localized prostate cancer to 70–78 Gy (2 Gy/fraction). Factor analysis was applied to previously identified PRO-based symptom domains from two study-specific questionnaires. Number of questions: 43; median time to follow-up: 3.6–6.4 years) and dose-response outcome variables were defined from these domains. Dose/volume parameters of the anal sphincter (AS) or the rectum were tested as predictors for each outcome variable using logistic regression with 10-fold cross-validation. Performance was assessed using area under the receiver operating characteristic curve (Az) and model frequency.
Results. Outcome variables from Defecation urgency (number of symptoms: 2–3), Fecal leakage (4–6), Mucous (4), and Pain (3–6) were defined. In both cohorts, intermediate rectal doses predicted Defecation urgency (mean Az: 0.53–0.54; Frequency: 70–75%), and near minimum and low AS doses predicted Fecal leakage (mean Az: 0.63–0.67; Frequency: 83–99%). In one cohort, high AS doses predicted Mucous (mean Az: 0.54; Frequency: 96%), whereas in the other, low AS doses and intermediate rectal doses predicted Pain (mean Az: 0.69; Frequency: 28–82%).
Conclusion. We have demonstrated that Defecation urgency, Fecal leakage, Mucous, and Pain following primary EBRT for localized prostate cancer primarily are predicted by intermediate rectal doses, low AS doses, high AS doses, or a combination of low AS and intermediate rectal doses, respectively. This suggests that there is a domain-specific dose-response for the GI tract. To reduce risk of GI morbidity, dose distributions of both the AS region and the rectum should, therefore, be considered when prescribing prostate cancer RT.
Current radiotherapy (RT) regimens for localized prostate cancer offer high survival rates with an expected 95% five-year survival [Citation1]. As 50% of the long-term survivors are expected to suffer from RT-induced adverse effects of the gastrointestinal (GI) tract, surveying GI morbidity and its influence on quality of life is important [Citation1,Citation2]. To reduce morbidity, a thorough understanding of the pathophysiology behind a certain RT-induced injury is required [Citation3], but many aspects of this complex puzzle still remains challenging.
GI morbidity following RT for prostate cancer involves distinct domains of symptoms related to, e.g. increased rates of bleeding, fecal frequency/leakage/urgency, or mucous loss [Citation3–5]. Patient- reported outcomes (PROs) have been shown to describe the relationship between GI morbidity and quality of life [Citation2], however, instruments for PROs are typically extensive and include symptoms from multiple domains. Reducing the dimension of PRO instruments according to a shared underlying cause between symptoms could potentially improve the understanding of domain-specific injuries [Citation6,Citation7]. Also, the impact of dose to all relevant structures should be identified [Citation4] and, for the GI tract, dose to individual segments might cause different domains of symptoms [Citation2,Citation5,Citation6,Citation8–11].
In previous work, we hypothesized that applying an unbiased method to group detailed patient-reported GI symptoms after RT for localized prostate cancer would provide new insights regarding distinct domains of RT-induced GI morbidity. Indeed, we observed four distinct domains of symptoms related to Defecation urgency, Fecal leakage, Mucous, or Pain in two Scandinavian prostate cancer studies (N = 1025) [Citation7,Citation12]. An important perspective of such symptom domains for radiation oncology is to investigate potential dose-response relationships. The aim of the current study was, therefore, to explore critical structure(s) for these four domains in patients treated with primary external-beam radiotherapy (EBRT) by studying whether dose to individual/combined segments of the GI tract could explain the occurrence and severity of domain-specific symptoms.
Material and methods
Information on the investigated Danish (DK) and Swedish (SW) cohorts that received primary EBRT for localized prostate cancer, and the GI symptom profiles is summarized below but further details can be found elsewhere [Citation13,Citation14].
Original study design, treatment, and risk structures
The DK study included 212 patients treated at Aarhus University Hospital, Aarhus in 2005–2007. The patients completed a Danish study-specific questionnaire (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779) in 2010 with a median time to follow-up (range) of 3.6 (2.4–5.0) years (Supplementary Table II, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779) [Citation14]. The average age (± SD) of the patients was 70 ± 5.0 years. The SW study consisted of 277 patients treated at Sahlgrenska University Hospital, Gothenburg in 1993–2006. The patients completed a second Swedish study-specific questionnaire (Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779) in 2008, and the median time to follow-up was 6.4 (1.2–14) years, and the average age 64 ± 5.0 years [Citation13].
EBRT for both cohorts was planned based on computed tomography (CT) imaging (patient in supine position) and prescribed according to the recommendations by ICRU [Citation15] with total doses of 70–78 Gy delivered in daily fractions of 2 Gy (five fractions/week). The majority of patients received conformal three-field treatments with 15 MV photon beam quality. In DK, the planning target volume (PTV) margin was 7 mm in all directions, except cranio-caudally where it was 9 mm. The majority of the patients in DK were treated with a two-phase approach including the proximal seminal vesicles in the first phase. In SW, the PTV margin was 20 mm in all directions, except posteriorly where the margin was the lesser of 15 mm or half of the rectal cross-sectional area.
For each patient, the anal sphincter region (including the anal canal, inner and outer sphincter muscles (AS)), and the rectum (from the slice above the anal canal to the slice below the recto-sigmoid flexure) were systematically delineated based on their outer borders.
Symptom domains and dose-response outcome variables
The GI symptom domains were identified using our factor analysis-based approach. Factor analysis is a statistical method to investigate the relationship between items in a dataset. A detailed explanation and related results can be found in the Appendix (available online at http://www.informahealthcare.com) and in [Citation7,Citation12]. In brief, we previously identified three domains (number of symptoms) in DK: Defecation urgency (3), Fecal Leakage (4), and Pain (6), and four domains in SW: Defecation urgency (2), Fecal leakage (6), Mucous (4), and Pain [Citation3] (Supplementary Figure 1, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779). Dose-response outcome variables were now defined as the resulting binary response when addressing the presence of symptoms in an identified domain (Boolean ‘OR condition’ was used when combining symptoms). We studied a moderate severity (typically occurring weekly and defined through discussions with M.H and D.A for DK and SW, respectively; ) where the prevalence of a symptom had to be ≥ 5% to be considered for further analyses.
Table I. The studied symptoms domains, symptom phrasing, moderate severity cut-off, and related proportions for both cohorts.
Dose-response modeling
The following dose/volume parameters of AS and rectal dose distributions were investigated as predictors for a certain symptom domain: the relative volume receiving xGy, Vx (V5–V80 and V5–V75 for DK and SW, respectively, in steps of 5 Gy), the minimum dose to the hottest x%, Dx, (D5–D100, in steps of 5%), the mean dose of the hottest x% volume, MOHx (MOH5–MOH100, in steps of 5%) as well as the maximum dose (Dmax) and the mean dose (Dmean).
Univariate analysis (UVA) using logistic regression with 10-fold cross-validation and 50 iterations was first applied to find the dose/volume parameters that predicted each symptom domain (potential candidates suggested by two-sided p-values < 0.10). In the subsequent multivariate analysis (MVA), we considered two approaches to carefully handle the number of important dose/volume parameters: a stepwise combined backward and forward selection, MVAstepwise and a Least Absolute Shrinkage and Selection Operator approach [Citation16], MVALASSO. Also these approaches were performed using 10-fold cross-validation with 50 iterations, and we report the area under the receiver operating curve (Az) and the frequency of the generated models. A certain dose/volume parameter was considered important if having a frequency > 5%. Any added predictability by inclusion of clinical parameters (age at RT (years), diabetes (yes/no), time to follow-up (years), hormonal treatment (yes/no; anti-androgens/gonadotropin-releasing hormone/orichiectomy), smoking (current vs. former/no), and structure volume (cm3); Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779) having a p-value < 0.10 on UVA was also investigated for each domain and more specifically for the combination of the most frequently occurring structure(s), dose(s), and symptom(s) with the highest Az. Corresponding structure dose distributions were ultimately investigated in a predictive normal tissue complication probability setting with the Lyman-Kutcher-Burman (LKB) model [Citation17–19]. We used the Maximum Likelihood method with a grid search approach (a range: 0.001:100 in 56 steps, D50 range: 50:150 in 101 steps, and m range: 0.01:1.1 in 55 steps) and the Profile Likelihood method for calculation of 95% confidence intervals (CIs).
Finally, we performed joint dose-response modeling across DK and SW for the combination of structures, and symptom(s) of specific domains with the highest predictability within each cohort following the above outlined procedures for UVA, and MVAstepwise MVALASSO. All analyses were performed in MATLAB v.R2013a.
Results
Dose-response modeling within cohorts
On UVA, intermediate rectal dose/volume parameters were individual predictors for Defecation urgency in both cohorts (included in 84–86% of the models; Supplementary Table III, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779). Individual dose/volume parameters for both AS and rectum predicted Fecal leakage (DK: AS included in 90% and rectum in 10% of the models; SW: AS and rectum included in 50% of the models), only high AS dose/volume parameters were individual predictors for Mucous in SW, and near minimum (SW) and low (DK) AS and rectal dose/volume parameters predicted Pain.
The two MVA approaches generated the same higher-frequency models, i.e. the same dose/volume parameters with equal discriminative ability (). However, MVALASSO provided one additional model (a Mucous model) and we hereafter, therefore, focus on the MVALASSO-generated models.
Table II. All MVA models with mean Az ≥ 0.50 sorted by the highest Az in each domain, and the dose/volume parameters with frequency > 5% for the models with the best discriminative ability (denoted by *).
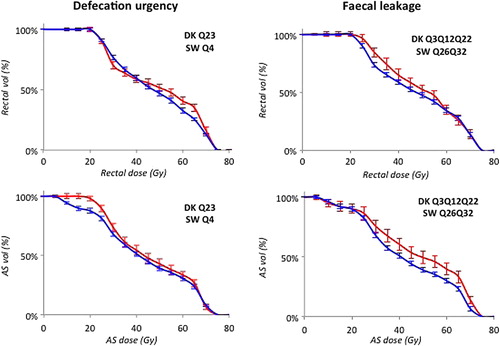
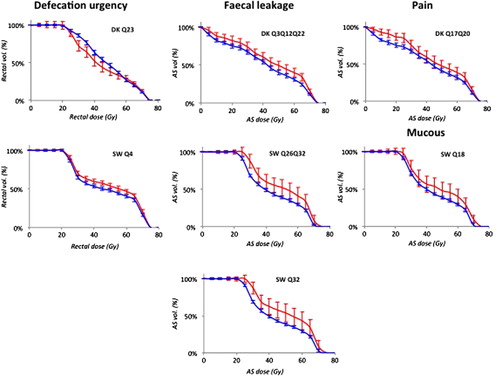
We identified one Defecation urgency model in DK (question/symptom id Q23; ) and one in SW (Q4) with similar discriminative ability (average Az: 0.54 and 0.53, respectively; ). In both cohorts, intermediate rectal doses predicted this domain (Frequency: 70–75% for D55 in DK and D40 in SW), whereas other intermediate and high rectal doses as well as near minimum and low AS doses had a considerably lower frequency. One Fecal leakage model was identified in DK (Q3Q12Q22) and two models in SW (1. Q26Q32 and 2. Q32). Similarly as for Defecation urgency, each Fecal leakage model's discriminative ability was in the same range for DK and SW (average Az: 0.62 and 0.67, respectively) but in contrast, near minimum and low AS doses instead predicted Fecal leakage (Frequency 10–99% for D100 in DK, and D95 and V30 in SW). One Mucous model including AS V70 (Frequency: 96%) was identified in SW (Q18) with a discriminative ability in the same range as for Defecation urgency (average Az: 0.54). One Pain model was identified in DK (Q17Q20) with a comparably high discriminative ability (average Az: 0.69) for both intermediate rectal and low AS doses (Frequency 82% and 28–68% for AS V15 and rectal V35–V40, respectively). The population averaged dose-volume histograms for patients with and without a moderate symptom severity used as input to these final models are illustrated in . For these final models, smoking was an individual predictor for Defecation urgency (SW: p = 0.03), whereas for Fecal leakage, age (DK: p = 0.07; SW: p = 0.02, 0.05), time to follow-up (DK: p = 0.01; SW: p = 0.10), AS volume (SW: p = 0.01, 0.04), and smoking (DK: p = 0.02) were individual predictors. Hormonal treatment (DK: p = 0.001) and smoking (DK: p = 0.02) predicted Pain. The added discriminative ability by including these clinical parameters to the final MVA models was modest with an increase in Az of 0.03 for Defecation urgency in SW, and decrease (DK)/increase (SW) of 0.01 in Az for Fecal leakage, and a 0.03 increase in Az for Pain in DK.
Figure 1. Population averaged dose-volume histograms for the highest frequency models of each symptom domain within the two cohorts (error bars denote 95% CI) for patients with < Moderate symptom severity (blue) and with ≥ Moderate symptom severity (red).
The value for the best-fitting LKB model parameter a suggested a serial behavior of rectum for Defecation urgency (a = 100 and 3 in DK and SW, respectively; ), and a more parallel behavior of AS for Fecal leakage (a = 0.01–0.03). For the Pain model in DK, the estimated a parameter values suggested a parallel behavior of AS and a serial behavior of rectum (AS: a = 0.09, rectal: a = 100), whereas the Mucous model in SW suggested a parallel behavior for AS (a = 0.50). The 95%CI of the ‘a’ value for all domains except SW and Defecation urgency were wide.
Table III. Best-fitting LKB model parameters for each symptom domain in DK, and in SW.
Dose-response modeling across cohorts
Outcome variables for the combined cohorts were defined from the MVA results. For Defecation urgency, DK Q23 was joined with SW Q4, and for Fecal leakage, DK Q3Q12Q22 was joined either with SW Q26Q32 or SW Q32 (; ). Compared to the separate cohort analyses, the discriminative ability was higher for Defecation urgency models (average Az: 0.62), whereas in the same range or lower for Fecal leakage models (average Az: 0.57–0.61). Intermediate rectal doses still predicted Defecation urgency (Frequency: 27% for Dmean) but near minimum AS doses had a considerably higher frequency (99% for D95). Low AS doses but also intermediate rectal doses predicted Fecal leakage (Frequency: 68% for AS V30 and 63% for rectal Dmean for the Fecal leakage model with the highest discriminative ability).
Discussion
Based on GI symptom profiles from two cohorts and two approaches to carefully handle the importance of GI tract dose/volume parameters, we found domain-specific dose-response relationships for each of the four investigated GI symptom domains. In particular, intermediate rectal doses were critical for Defecation Urgency, whereas near minimum and low AS doses were critical for Fecal leakage. High AS doses were important for Mucous, and both low AS doses and intermediate rectal doses were important for Pain.
RT for any tumor site usually involves a trade-off between tumor eradication and the risk of developing morbidity, where the former will always have a high priority. Avoiding morbidity in non-tumor bearing tissues after RT for prostate cancer is, particularly, motivated provided the high survival rates. The primary focus is to avoid GI morbidity since it has been reported to impact quality of life more than sexual or urinary morbidity [Citation2]. To avoid GI morbidity after RT for localized prostate cancer, the traditional approach has been to limit the dose to the rectum [Citation4], which also applies to the subjects included in this study. Such precautions are less recognized for the AS. From objective measures of rectal and anal contractility/pressure after RT for prostate cancer, decreased rectal capacity, reduced maximum tolerated rectal distension [Citation3], and reduced pressure by the internal and the external ASs have been reported [Citation3,Citation9]. From physician-based scorings of morbidity, relationships between dose to the superior parts of the rectum and mucous or urge without stools have previously been observed, whereas dose to the inferior parts of the rectum has been shown to predict soiling or fecal incontinence [Citation10].
For our PRO-based data, intermediate rectal doses predicted Defecation urgency but when combining the cohorts and joining our two Defecation urgency domains, also lower doses to the AS predicted this symptom domain. This is line with the results by Smeenk et al. [Citation5] who, although using a more general defecation urgency definition (‘Urgency present, no therapy’), found that doses to both the rectum and the anal canal were critical for this condition. Recently, Ebert et al. observed that low to intermediate doses to the anal canal predicts defecation urgency in combination with tenesmus [Citation20]. In our data, intermediate doses to the rectum in combination with low doses to the AS were critical for Defecation urgency and Pain. Regarding Fecal leakage, our findings are in line with those of Smeenk et al. [Citation5] and Vordermark et al. [Citation6] who found that low doses to the anal canal predicted specific fecal leakage symptoms after RT for localized prostate cancer (‘Incontinence present, no therapy’ in [Citation5] and ‘Sometime incontinent for solid stools’ in [Citation6]). The Fecal leakage models with the best discriminative ability in our study included up to three symptoms, whereas Vordermark et al. did not find any dose correlation combining the 10 investigated items. This might imply that a more sophisticated approach of combining symptoms, as demonstrated in our study, is needed to predict this condition but we cannot exclude ambiguities between studies due to the substantially smaller single-institutional cohort investigated in [Citation6]. For a large cohort, Peeters et al. [Citation11] found that low, intermediate, as well as high doses to the anus predicted incontinence (‘use of pads > twice/week’). For the comparable symptom on pad use in SW (Q32), and for the remaining Fecal leakage domains, we found only low to intermediate doses of the AS to be critical. However, since the anus was defined as the most caudal 3 cm of the rectum in [Citation11], it is likely that the correlations with the various doses of the anal canal better corresponds to dose/volume parameters of the rectum in our study. Indeed, for the combined cohorts we found that models including the rectal mean dose were comparable in frequency to models including lower AS doses.
Few studies have investigated LKB model parameters for general GI symptoms. Gulliford et al. estimated parameters for the rectum only [Citation21], Defraene et al. studied the anal wall [Citation22], whereas Peeters et al. studied both the rectum and the anal canal (walls) [Citation23]. A comparison with the results from these studies is possible for our Defecation urgency and Fecal leakage domains (Rectal urgency’ in [Citation21] and ‘Fecal incontinence’ in [Citation22,Citation23], respectively). For our combined cohorts, the LKB parameters suggested a slightly more serial behavior for the rectum and Defecation urgency (a = 8 vs. 3), higher D50 values (79 vs. 68 Gy) and a somewhat more shallow dose-response curve (m = 0.43 vs. 0.36) than Gulliford et al. For Fecal leakage, the values of a diverged (a = 13 or 100 vs. 1 [Citation22] or 0.13 [Citation23]), the D50 values somewhat (150 vs. 105 Gy [Citation22,Citation23]) but the steepness of the dose-response curve was similar (m = 0.47 or 0.51 vs. 0.42 [Citation22] or 0.36 [Citation23]). These differences must be interpreted in light of differences such as morbidity assessment and outcome variable definition (single physician-assessed symptoms were used in all three studies), as well as structure definitions.
A strength of this study is that the results were based on two large prostate cancer cohorts where dose-volume response relationships between different segments of the GI tract and specific GI symptom domains could be thoroughly investigated. We applied two independent statistical methods to describe relationships between structure(s), dose(s), and symptom(s) with both methods arriving at the same best-fitting models. The focus on the high rectal doses to avoid GI morbidity has typically been preceded by studies reporting on rectal bleeding or GI morbidity as assessed by combining symptoms from different domains using physician-based scoring systems. Although the highest impact on QOL has been observed for defecation urgency and fecal leakage [Citation2], these domains have typically not been investigated to the same extent as here. For instance, only one commonly used scoring system includes either of these domains (EPIC [Citation24] and CTCAE [Citation25], respectively) but defecation urgency in EPIC is scored in combination with pain and the level of detail for fecal leakage in CTCAE is coarser than for any of the symptoms in our Fecal leakage domain. The prevalence of moderate rectal bleeding symptoms in our series was low (< 6% for all concerned symptoms), and these symptoms did not form a specific symptom domain. We, therefore, did not study rectal bleeding and our results suggest that this domain is better studied in isolation. Addressing generalizability of the identified dose-volume response relationships with respect to clinical parameters indicated a typically modest increase in the discriminative ability, with the Az value at most increasing 0.03 for smoking in Defecation urgency in SW (Az: 0.53 vs. 0.56), as well as for hormonal treatment and smoking in Pain for DK (Az: 0.69 vs. 0.72). Time to follow-up was an individual predictor for Fecal leakage in both cohorts but did not add substantially to the discriminative ability. Many of the GI symptoms, as presented for the SW cohort [Citation26], have previously been reported to have a stable temporal pattern and, therefore, it is not surprising that our final models presented here are not highly time dependent. In the Swedish study, we had access to a reference cohort from the general population where symptom background rates had been assessed. For the symptoms included in our final models, the symptom rate in survivors was at least twice the symptom rate in the reference cohort (data not shown) indicating that our factor analysis approach indeed is able to detect RT-induced symptoms. Ideally such an internal check should have been performed for both cohorts, and this needs be kept in mind when interpreting the results. Finally, cross-validation typically yields more robust results than results by a single regression analysis, which may result in models with a lower discriminative ability. Models having Az near 0.50 are regarded as not being better than random chance. The Az for our final models ranged between 0.51–0.67 and this, of course, limits the clinical usefulness for the weaker models at this stage. However, our overall proposed approach assists in identifying the more prominent symptoms within a symptom domain and if these can be avoided in RT, maybe the occurrence of related symptoms can be reduced. This alone merits the further exploration of our results in advancing our understanding of the complex interplay between GI symptoms after prostate cancer RT.
In conclusion, knowledge about when to address single symptoms and when to address multiple symptoms to avoid RT-induced morbidity is limited. We have used an exploratory approach to investigate such relationships between dose to single structures of the GI tract and single as well as multiple GI symptoms and demonstrated that dose to the rectum is critical for Defecation urgency, whereas dose to the AS plays a key role for Fecal leakage and Mucous (low and high dose, respectively). The corresponding pattern for Pain was identified from a combination of low dose to the AS and intermediate dose to the rectum. This suggests that there is a domain-specific dose-response for the GI tract and, therefore, dose distributions of both the AS region and the rectum should be considered when prescribing prostate cancer RT. As the AS is situated further away from the prostate than the rectum, and in analogy with the previously suggested AS-sparing surgery as proposed by Gervaz et al. [Citation9], sphincter-preserving prostate RT should be feasible and could be appropriate for the purpose of further understanding and reducing multiple GI symptoms in these patients.
Supplementary material available online
Supplementary Appendix, Supplementary Figure 1 and Tables I–III available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063779
ionc_a_1063779_sm7771.ps
Download (80.7 KB)Acknowledgments
This work was supported by the Swedish Cancer Society, the King Gustav V Jubilee Clinic Cancer Foundation in Göteborg, the Swedish state under the ALF agreement in Göteborg and Stockholm and Varian Corporation. The Assar Gabrielsson Foundation, Tore Nilssons Foundation, and “Syskonen Svenssons fond för medicinsk forskning” are also gratefully acknowledged.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: A new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007–17.
- Krol R, Smeenk RJ, van Lin EN, Hopman WP. Impact of late anorectal dysfunction on quality of life after pelvic radiotherapy. Int J Colorectal Dis 2013;28:519–26.
- Krol R, Hopman WP, Smeenk RJ, Van Lin EN. Increased rectal wall stiffness after prostate radiotherapy: Relation with fecal urgency. Neurogastroenterol Motil 2012;24:339–e166.
- Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 2010;76:S123–9.
- Smeenk RJ, Hopman WP, Hoffmann AL, van Lin EN, Kaanders JH. Differences in radiation dosimetry and anorectal function testing imply that anorectal symptoms may arise from different anatomic substrates. Int J Radiat Oncol Biol Phys 2012;82:145–52.
- Vordermark D, Schwab M, Ness-Dourdoumas R, Sailer M, Flentje M, Koelbl O. Association of anorectal dose-volume histograms and impaired fecal continence after 3D conformal radiotherapy for carcinoma of the prostate. Radiother Oncol 2003;69:209–14.
- Olsson CE, Thor M, Oh JH, Elleberg Petersen D, Alsadius D, Høyer M, et al. Is the anal sphincter a key structure for gastrointestinal toxicity in prostate cancer radiotherapy? Radiother Oncol 2015;115:S467.
- Buettner F, Gulliford SL, Webb S, Sydes MR, Dearnaley DP, Partridge M, The dose-response of the anal sphincter region – an analysis of data from the MRC RT01 trial. Radiother Oncol 2012;103:347–52.
- Gervaz PA, Wexner SD, Pemberton JH, Pelvic radiation and anorectal function: Introducing the concept of sphincter-preserving radiation therapy. J Am Coll Surg 2002;195:387–94.
- Heemsbergen WD, Hoogeman MS, Hart GA, Lebesque JV, Koper PC. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:1011–8.
- Peeters ST, Lebesque JV, Heemsbergen WD, van Putten WL, Slot A, Dielwart MF, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2006;64:1151–61.
- Thor M, Olsson CE, Oh JH, Petersen SE, Alsadius D, Høyer M, et al. Identifying groups of patient-reported gastro-intestinal symptoms using factor analysis. Int J Radiat Oncol Biol Phys 2014;90:S854.
- Alsadius D, Hedelin M, Johansson KA, Pettersson N, Wilderang U, Lundstedt D, et al. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol 2011;101:495–501.
- Petersen SE, Bentzen L, Emmertsen KJ, Laurberg S, Lundby L, Hoyer M, Development and validation of a scoring system for late anorectal side-effects in patients treated with radiotherapy for prostate cancer. Radiother Oncol 2014;111:94–9.
- International Commissions on Radiation Units and Measurements (ICRU) I. (Supplement to ICRU Report 50). Prescribing, recording and reporting photon beam therapy. Bethesda, MD: ICRU; 1999.
- Tibshirani R, Regression shrinkage and selection via the Lasso. J Royal Stat Soc 1996;58:267.
- Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R, Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys 1991;21:137–46.
- Lyman JT, Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13–9.
- Niemierko A, Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys 1997; 24:103–10.
- Ebert MA, Foo K, Haworth A, Gulliford SL, Kennedy A, Joseph DJ, et al. Gastrointestinal dose-histogram effects in the context of dose-volume-constrained prostate radiation therapy: Analysis of data from the RADAR prostate radiation therapy trial. Int J Radiat Oncol Biol Phys 2015;91:595–603.
- Gulliford SL, Partridge M, Sydes MR, Webb S, Evans PM, Dearnaley DP. Parameters for the Lyman Kutcher Burman (LKB) model of Normal Tissue Complication Probability (NTCP) for specific rectal complications observed in clinical practise. Radiother Oncol 2012;102:347–51.
- Defraene G, Van den Bergh L, Al-Mamgani A, Haustermans K, Heemsbergen W, Van den Heuvel F, et al. The benefits of including clinical factors in rectal normal tissue complication probability modeling after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:1233–42.
- Peeters ST, Hoogeman MS, Heemsbergen WD, Hart AA, Koper PC, Lebesque JV. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: Normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys 2006;66:11–9.
- Chang P, Szymanski KM, Dunn RL, Chipman JJ, Litwin MS, Nguyen PL, et al. Expanded prostate cancer index composite for clinical practice: Development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol 2011;186:865–72.
- The National Cancer Institute. Common Terminology Criteria for Adverse Events v4.03 (NCI CTCAE v4.03). 2010.
- Alsadius D, Olsson C, Pettersson N, Tucker SL, Wilderang U, Steineck G. Patient-reported gastrointestinal symptoms among long-term survivors after radiation therapy for prostate cancer. Radiother Oncol 2014;112:237–43.