ABSTRACT
Background. Residual neck disease after radiotherapy in advanced oropharyngeal squamous cell carcinoma (OPSCC) is associated with increased mortality, and some patients may benefit from post-radiotherapy neck dissection (PRND). The aim of the present study was to assess the value of magnetic resonance imaging (MRI) and other clinical characteristics in selecting patients for PRND.
Materials and methods. Retrospective cohort study. Consecutive patients with N+ OPSCC were included. Medical records, pathology reports and imaging reports were reviewed. Pre- and post-therapeutic imaging was re-evaluated.
Results. A total of 100 consecutive patients from a three-year period were included. Neck response was evaluated with MRI two months after treatment. Sixty patients were suspicious for residual neck disease, and were offered surgery; seven of these patients had histologic evidence of carcinoma. Cumulative neck failure after three years was 14% (8.4–24%), and did not differ significantly among patients with positive compared to negative MRI (radiologist's initial description; p = 0.47, log-rank test). Applying neck failure as gold standard, sensitivity and specificity of MRI was 69% and 41%, respectively; positive and negative predictive value was 15% and 90%. Patients with p16 + disease had significantly larger lymph nodes after treatment, and imaging based on lymph node size resulted in many false positives. Analysis of receiver operating characteristic curves in 191 individual lymph nodes showed that a short axis ≥ 10 mm should be classified as suspicious. Furthermore, T-stage and p16-status were associated with increased risk of neck recurrence. Salvage was successful in four patients with early detected nodal recurrence.
Conclusion. These results suggest that lymph node size, T-stage and p16 status could be used in selecting patients for PRND in OPSCC. Yet, early anatomical imaging may be inappropriate for evaluating neck response in patients with p16 + disease as enlarged lymph nodes often do not indicate residual neck disease.
Management of the neck after curatively intended radiotherapy of advanced head and neck squamous cell carcinoma (HNSCC) has long been a matter of debate. Residual neck disease is associated with worse overall and disease-free survival, and so patients with evidence of residual neck disease may benefit from post-radiotherapy neck dissection (PRND) [Citation1]. Response rates are high after chemoradiation even in advanced HNSCC. Accordingly, most authors argue that only patients with suspected residual or recurrent disease should be offered PRND, while patients with a complete neck response should be carefully observed [Citation2,Citation3].
The rising incidence of HPV-associated oropharyngeal squamous cell carcinoma (OPSCC) has changed the epidemiology dramatically [Citation4,Citation5]. Patients with HPV-associated OPSCC typically have more advanced nodal disease, yet respond better to radiotherapy [Citation6,Citation7]. As a consequence, we are treating an increasing number of patients with a reduced need for PRND. Accurately selecting patients who may benefit from this additional treatment is thus of increasing importance.
At our institution treatment response is evaluated with magnetic resonance imaging (MRI) two months after radiotherapy. The aim of this study was to assess the value of MRI for response evaluation of metastatic neck lymph nodes after curative radiotherapy in patients with node-positive OPSCC. A secondary aim was to explore clinical and radiological features predictive of residual neck disease with special emphasis on the importance of HPV status.
Material and methods
Clinical data
In this retrospective cohort study we reviewed medical records, pathology reports and imaging reports of consecutive patients registered with a diagnosis of OPSCC in the years 2010–2012. We excluded patients who did not have curatively intended radiotherapy, who did not have MRI for response evaluation, who had a neck dissection before radiotherapy or patients with less than one year of follow-up. Demographic data, disease characteristics, imaging results and outcome data were registered. Patients were staged according to TNM classification based on clinical and radiological data. Tumour tissue was routinely examined for p16 using standard immunohistochemistry, and considered positive if more than 70% of tumour cells showed strong and uniform cytoplasmic and nuclear staining [Citation8].
Imaging
Original imaging reports were reviewed and classified as either negative if no evidence of residual neck disease was noted by the radiologist or as positive if suspicion was noted.
For purpose of the present study, pre- and post-therapy scans were re-examined by two radiologists blinded to patient outcome (authors VFJ and HWE). A maximum of three lymph nodes per scan were measured in three dimensions and volume estimated as long axis * short axis * third axis * π/6.
Statistical analysis
The primary endpoint was histologically confirmed neck recurrence. Diagnostic accuracy of MRI for detection of residual neck disease was estimated by comparing the original description dichotomised as either positive or negative with histologically confirmed neck recurrence in the course of follow-up as gold standard.
Time-to-event data were analysed from date of response evaluation, and failure recorded in the event of neck recurrence. Local recurrence, distant failure, diagnosis of new head and neck cancer or death was considered censoring events. Cumulative neck failure was estimated using Kaplan-Meier method, and compared using log-rank test.
Categorical variables were compared using Fisher's exact test or χ2-test in case of more than two categories. Continuous variables were compared with Wilcoxon rank-sum test.
Correlation of various lymph node measurements with neck failure on a single-node basis was analysed using receiver operating characteristics (ROC) curves. Optimal cut-off for each variable was chosen with a minimum sensitivity of 80%.
Results were considered statistically significant at p-values less than 5%, and 95% confidence interval (CI) was calculated for point estimates where possible. Statistical analyses were performed using Stata 13.1 (StataCorp, TX, USA).
Results
Patient characteristics and treatment
We identified a total of 207 consecutive patients diagnosed with OPSCC at Aarhus University Hospital in the three years of whom 177 had node-positive disease at diagnosis. Another 77 patients were excluded as indicated in Supplementary Figure 1 (available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063781). In total, 100 patients were included in the study. Patient, tumour and treatment characteristics are summarised in .
Table I. Patient, tumour and treatment characteristics (n = 100).
Response evaluation and MRI
All patients had an MRI for response evaluation at a median of 7.9 weeks after last fraction of radiotherapy (range 6.1–11 weeks). Patients had MRI at five different institutions and imaging was interpreted by a larger number of radiologists. Based on the original description no evidence of residual neck disease was noted on MRI in 40 patients, and were thus classified as negative for this study. In the remaining 60 patients residual neck disease was suspected and was classified as positive.
Following MRI, patients were seen at a multidisciplinary team conference and evaluated clinically with 60 patients subsequently undergoing neck surgery. In most patients (n = 38) surgery consisted of excision of suspicious lymph nodes with immediate frozen section histology, and patients would then undergo neck dissection of adjacent lymph node levels if histology were positive for carcinoma. A minority of the surgically treated patients had a selective neck dissection without prior histologic evidence of residual neck disease (n = 22).
The remaining 40 patients were followed clinically as per DAHANCA guidelines with imaging only on clinical indication. Median recurrence-free follow-up was 2.9 years (range 1.0–4.4 years). One patient developed a new primary head and neck cancer (floor of mouth) and was censored after 20 months.
Outcome
Of the 60 patients undergoing surgery immediately after response evaluation seven patients had histologically confirmed residual neck disease (12%, 95% CI 4.8–23%). Two patients had a neck recurrence during follow-up despite initially negative histology; one was an isolated recurrence, the other had simultaneous distant failure.
Of the 40 patients who were followed without PRND, four patients developed a neck recurrence during follow-up resulting in a cumulative neck failure after three years of 10% (3.9–25%). Two patients had isolated nodal recurrence; the other two patients had simultaneous local or distant recurrence.
Overall, a total of 13 patients developed a neck recurrence during the course of follow-up. Estimated cumulative neck failure at three years was 14% (95% CI 8.4–24%). Ten patients had isolated nodal recurrence, one patient had a simultaneous T-site recurrence and two patients had nodal recurrence combined with distant failure (see Supplementary Figure 2, available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063781).
Diagnostic accuracy of MRI
Comparing radiologists original description of MRI with a gold standard of histologically confirmed neck recurrence we found that MRI correctly identified nine of 13 neck recurrences, yielding a sensitivity of 69% (95% CI 39–91%). However, there was a high rate of false positive scans and so specificity and positive predictive value was low. Overall, accuracy was 45% (35–55%, ).
Table II. Diagnostic accuracy of radiologist's initial description of MRI for response evaluation two months after radiotherapy in OPSCC.
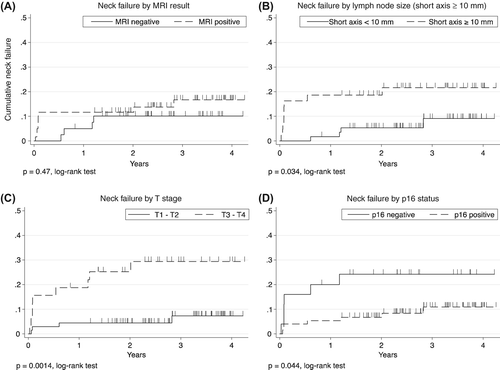
Estimated cumulative neck failure after three-years was 17% (8.9–30%) among patients with positive MRI compared to 10% (3.9–25%) among patients with negative MRI (p = 0.47, log-rank test, ).
Lymph node measures
To evaluate which lymph node measures were the most predictive of residual neck disease we explored measures of short axis, long axis and volume in both pre- and post-therapeutic scans, and compared this to a gold standard of neck recurrence on a single-node basis.
A total of 191 lymph nodes were measured after treatment, compared to 189 before treatment (pre-therapeutic scans were unavailable in a few patients). There were a total of 11 nodal recurrences in corresponding neck levels as the remaining two neck failures in the cohort occurred in neck levels with no corresponding lymph nodes at the time of MRI.
Measurements of lymph node size (short axis, long axis or volume) in both pre- and post-therapeutic scans all held significant diagnostic information with area under ROC curves significantly larger than 0.5. The single most informative measurement was short axis in the post-therapeutic scan with a cut-off at ≥ 10 mm being the most optimal ().
Table III. Lymph node measurements and association with recurrence in 191 single lymph nodes.
The degree of lymph node resolution as indicated by the ratio of post- to pre-therapeutic measurements or volume was not informative. The shape of the lymph node as indicated by the ratio of short axis to long axis measurement (known as Solbiati index) also did not provide useful diagnostic information.
Other factors associated with neck recurrence
We found that p16-negative disease, T-stage and having a lymph node with a short axis measurement ≥ 10 mm was associated with neck recurrence (, ). Higher N-stage was not associated with increased risk. Unfortunately, the small number of events did not allow for multivariate analysis. Diagnostic accuracy of MRI, if a criterion of short axis ≥ 10 mm is applied, is indicated in Supplementary Table I, available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063781.
Table IV. Various factors predicting neck recurrence.
Influence of p16 status
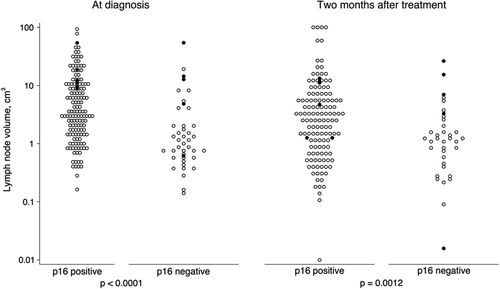
Patients with p16-positive disease had much larger lymph nodes at diagnosis with a median volume of 3.5 cm3 versus 1.0 cm3 for p16-negative (p < 0.0001, ).
At response evaluation two months after radiotherapy patients with p16-positive disease still had significantly larger lymph node volume, 0.60 cm3 versus 0.28 cm3 (p = 0.0012).
When applying a short axis measure of ≥ 10 mm as criterion for a positive test there are significantly more false positives among p16-positive patients (p = 0.001). As a consequence, this test is much less specific among p16-positive patients with a specificity of 53% (95% CI 40–65%) among patients with p16-positive disease versus 90% (95% CI 67–99%) for p16-negative (Supplementary Table I, available online at http://www.informahealthcare.com/doi/abs/ 10.3109/0284186X.2015.1063781).
Salvage results
Of the 13 patients with nodal recurrence, 10 patients had isolated nodal recurrence; four have been salvaged successfully, and remain alive and disease-free at last follow-up. All four successfully salvaged patients had an early nodal recurrence, detected at the planned response evaluation and diagnosed 66–79 days after end of radiotherapy. Thus, four of seven recurrences detected earlier than 100 days after end of treatment were successfully salvaged while none of the five patients with later recurrences could be salvaged successfully (p = 0.081).
Discussion
In this retrospective cohort study, we show that early anatomical MRI was unreliable for detecting residual nodal disease. Applying a strict criterion of short axis measure of ≥ 10 mm for classifying MRI as positive for residual neck disease added significantly to diagnostic accuracy. High T-stage and negative p16 status were also significantly associated with increased risk of neck recurrence during follow-up in a univariate analysis.
We also found that patients with p16-positive disease had much larger lymph nodes after radiotherapy, and that applying a geometrical criterion, such as short axis measurement, leads to very low specificity in these patients.
Salvage was only successful in patients with early-detected nodal recurrences, whereas salvage was invariably unsuccessful in patients with nodal recurrence detected later than 100 days after end of treatment.
A major limitation of this study is the small size and the low number of events in this group of patients with a favourable prognosis. Also, not all patients received surgery after chemoradiation; and so treatment, as well as histological evidence of treatment response, differs among patients. The majority of surgically treated patients only had a lymph node excision performed which increases the risk that a truly residual cancer was missed. Moreover, prior studies have indicated that residual carcinoma in neck dissection specimens following radiotherapy may not always be biologically viable, and so neck failure rate may be overestimated among surgically treated patients [Citation9]. Furthermore, it is possible that not all patients in this cohort had truly node-positive disease, as this classification was based on clinical and radiological findings. Recently, increased use of PET imaging has been shown to lead to significant stage migration [Citation10]. Strengths of the study include the consecutive and well characterised patient cohort with very homogenous diagnosis and treatment.
In advanced HNSCC, residual nodal disease is associated with adverse outcome [Citation1]. Most authors agree that PRND improves prognosis in patients with residual neck disease, but management is controversial due to lack of evidence from randomised or even prospective trials [Citation11]. As a consequence, management is mainly supported by historical data from an era when HPV-associated disease was less prevalent, and chemoradiation strategies significantly different.
In OPSCC, epidemiology and prognosis has transformed substantially in recent years due to the rapidly rising incidence of HPV-associated disease [Citation4–6]. These patients are more likely to have advanced-stage, node-positive disease [Citation7].
Neck control rates after radiotherapy in OPSCC has historically been in the range of 70–75% after five years depending on various prognostic factors [Citation12,Citation13]. As a consequence of altered biology and modern chemoradiotherapy with improved fractionation schemes and delivery techniques, loco-regional control rates are now very high in patients with OPSCC estimated at 88% after five years in the DAHANCA-18 trial [Citation14].
With loco-regional control rates of this order, most authors do not find routine PRND warranted instead recommending surgery to patients with clinical or radiological suspicion of residual neck disease [Citation2,Citation3,Citation11,Citation15]. This is based on the fact, that neck dissection bears risk of complications, such as wound complications, nerve injury or other complications related to surgical procedures; all of which may be more prevalent after radiotherapy [Citation16,Citation17]. However, it has been said that recurrence is the worst complication, and this remains true in this context, as several authors have found that late neck recurrences rarely are possible to salvage which is in line with our findings [Citation18].
Altogether, an increasing number of patients with advanced neck disease are being treated, but a decreasing proportion of these patients will have residual neck disease after initial treatment. Thus, it is of growing importance to accurately identify patients who may benefit from PRND. Many questions are unresolved including imaging modalities and timing.
Our results indicate that MRI may be used in this setting if a strict criterion of a short axis measurement of ≥ 10 mm is used to indicate suspicious lymph nodes. This is in line with the 2009 update of Response valuation Criteria in Solid Tumours (RECIST) in which measurement of lymph node short axis now is standard when evaluating treatment response in clinical trials. A reduction in short-axis to ˂10 mm is now considered a complete response in this regard [Citation19].
However, our results show that patients with p16-positive disease have larger lymph nodes following radiotherapy, and as these patients respond much better to therapy, enlarged lymph nodes may not necessarily harbour viable cancer cells. A similar study found that lymph nodes in patients with p16-positive disease take longer time to regress completely [Citation20]. Consequently, early imaging relying on measures of lymph node size leads to less specificity and a higher rate of unnecessary surgery in patients with p16-positive disease.
Disease stage or biological characteristics may also be used to estimate the risk of treatment failure. We found that T stage and p16 status were associated with increased risk of neck failure which is in line with previous findings [Citation7]. The question is then, which patients should be offered PRND – do we rely on imaging, clinical evaluation, biomarkers, disease stage or other characteristics?
In our cohort, we observed a three-year neck failure rate of 11% among patients with p16-positive disease. This would seem too low to warrant PRND in these patients as the excess morbidity would likely outweigh the modest benefit. In these patients, it would seem logical to reserve PRND to patients with clinical or radiological signs of residual nodal disease.
Among patients with p16-negative disease, the three-year risk of neck recurrence was doubled at 24%. This could justify offering PRND to all patients with p16-negative disease regardless of clinical or radiological assessment of treatment response. However, in our series the diagnostic value of imaging was much higher among patients with p16-negative disease which could justify a strategy of offering PRND only to patients with radiological evidence of residual nodal disease. These findings underline that future studies on management of the neck in OPSCC should distinguish between HPV-positive and -negative disease.
Other imaging modalities may also be considered in this setting. Ultrasound with fine-needle aspiration cytology has been evaluated in a minor cohort of patients, and was found to have rather low accuracy, possibly because of chemoradiotherapy effects on cytopathological interpretation [Citation21]. Also, this modality gives no information on T-site. Numerous smaller studies have shown promise for positron emission tomography (PET)/computed tomography (CT) imaging in this setting with very high negative predictive value, further information on the precise role of this modality is expected with the UK PET-NECK trial [Citation22–24].
Optimal timing of imaging for response evaluation remains controversial. Diagnostic accuracy will invariably increase with time in this setting as is often demonstrated [Citation23,Citation25]. However, findings from the present and other studies clearly indicate that early salvage is more successful. Quite possibly, timing of imaging should depend on p16 status with delayed imaging being more suitable in patients with p16-positive disease to allow enlarged but benign lymph nodes to regress.
In summary, this study suggests that MRI has limited value in selecting patients for PRND in OPSCC if not applying strict response evaluation criteria. Other prognostic factors, such as T-stage and p16 status, may be equally important.
The fact that patients with p16-positive disease tend to have larger lymph nodes after radiotherapy, while still having the most favourable prognosis, negatively affects the value of a diagnostic test based on early measurement of lymph node size in these patients.
Supplementary material available online
Supplementary Figure 1, 2 and Table I available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1063781.
ionc_a_1063781_sm1124.pdf
Download PDF (186.5 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sandulache VC, Ow TJ, Daram SP, Hamilton J, Skinner H, Bell D, et al. Residual nodal disease in patients with advanced-stage oropharyngeal squamous cell carcinoma treated with definitive radiation therapy and posttreatment neck dissection: Association with locoregional recurrence, distant metastasis, and decreased survival. Head Neck 2013;35:1454–60.
- Ferlito A, Corry J, Silver CE, Shaha AR, Thomas Robbins K, Rinaldo A. Planned neck dissection for patients with complete response to chemoradiotherapy: A concept approaching obsolescence. Head Neck 2010;32:253–61.
- Hermann RM, Christiansen H, Rödel RM. Lymph node positive head and neck carcinoma after curative radiochemotherapy: A long lasting debate on elective post-therapeutic neck dissections comes to a conclusion. Cancer Radiother 2013;17:323–31.
- Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol 2010;95:371–80.
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–301.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35.
- Lassen P, Primdahl H, Johansen J, Kristensen CA, Andersen E, Andersen LJ, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol 2014;113:310–6.
- Lassen P, Overgaard J. Scoring and classification of oropharyngeal carcinoma based on HPV-related p16-expression. Radiother Oncol 2012;105:269–70.
- Strasser MD, Gleich LL, Miller MA, Saavedra HI, Gluckman JL. Management implications of evaluating the N2 and N3 neck after organ preservation therapy. Laryngoscope 1999;109:1776–80.
- VanderWalde N, Salloum RG, Liu T-L, Hornbrook MC, O’Keeffe Rosetti MC, Ritzwoller DP, et al. Positron emission tomography and stage migration in head and neck cancer. JAMA Otolaryngol Neck Surg 2014;140:654.
- Mendenhall WM, Villaret DB, Amdur RJ, Hinerman RW, Mancuso AA. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck 2002;24:1012–8.
- Johansen LV, Grau C, Overgaard J. Squamous cell carcinoma of the oropharynx – an analysis of treatment results in 289 consecutive patients. Acta Oncol 2000;39:985–94.
- Johansen LV, Grau C, Overgaard J. Nodal control and surgical salvage after primary radiotherapy in 1782 patients with laryngeal and pharyngeal carcinoma. Acta Oncol 2004;43:486–94.
- Bentzen J, Toustrup K, Eriksen JG, Primdahl H, Andersen LJ, Overgaard J. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol 2015;54:1001–7.
- Van der Putten L, van den Broek GB, de Bree R, van den Brekel MWM, Balm AJM, Hoebers FJP, et al. Effectiveness of salvage selective and modified radical neck dissection for regional pathologic lymphadenopathy after chemoradiation. Head Neck 2009;31:593–603.
- Davidson BJ, Newkirk KA, Harter KW, Picken CA, Cullen KJ, Sessions RB. Complications from planned, posttreatment neck dissections. Arch Otolaryngol Head Neck Surg 1999;125:401–5.
- Genden E, Ferlito A, Shaha A, Talmi Y, Robins K, Rhys-Evans P, et al. Complications of neck dissection. Acta Otolaryngol 2003;123:795–801.
- Mabanta SR, Mendenhall WM, Stringer SP, Cassisi NJ. Salvage treatment for neck recurrence after irradiation alone for head and neck squamous cell carcinoma with clinically positive neck nodes. Head Neck 1999;21:591–4.
- Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer 2009;45:261–7.
- Huang SH, O’Sullivan B, Xu W, Zhao H, Chen DD, Ringash J, et al. Temporal nodal regression and regional control after primary radiation therapy for N2-N3 head- and-neck cancer stratified by HPV status. Int J Radiat Oncol Biol Phys 2013;87:1078–85.
- De Bree R, van der Putten L, Brouwer J, Castelijns JA, Hoekstra OS, Leemans CR. Detection of locoregional recurrent head and neck cancer after (chemo)radiotherapy using modern imaging. Oral Oncol 2009;45:386–93.
- Gupta T, Jain S, Agarwal JP, Rangarajan V, Purandare N, Ghosh-Laskar S, et al. Diagnostic performance of response assessment FDG-PET/CT in patients with head and neck squamous cell carcinoma treated with high-precision definitive (chemo)radiation. Radiother Oncol 2010; 97:194–9.
- Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol 2008;33:210–22.
- Ampil F, Caldito G, Reiser C, Devarakonda S, Takalkar A, Nathan C-A, et al. The prognostic utility of 18 F-FDG-PET metabolic tumor response after chemoradiotherapy for locally advanced head and neck cancer. Acta Oncol 2015;54: 1066–7.
- Tan M. Timing of restaging PET/CT and neck dissection after chemoradiation for advanced head and neck squamous cell carcinoma. Otolaryngology 2013;3:1–6.