ABSTRACT
Background. To assess planning aims (PAs) and dose prescription in image-guided adaptive brachytherapy (IGABT) of cervical cancer and investigate potential impact on clinical outcome.
Material and methods. Our study population consists of 225 consecutive cervical cancer patients (FIGO stages IB–IVA) treated between 1998 and 2008 at the Medical University of Vienna by external beam radiotherapy (EBRT) ± chemotherapy and IGABT. For this retrospective study, patients were stratified into two treatment groups: PA+ group, all dose constraints fulfilled for prescription; PA-, one or more dose constraints not fulfilled for prescription. The following dose constraints (EBRT+ IGABT) were applied: clinical target volume (CTV)HR D90 ≥ 85 Gy, D2cm3 Rectum < 70 Gy, D2cm3 Bladder < 90 Gy. Differences in patient, tumor and treatment characteristics and clinical outcome (event: local failure or grade 3 + 4 toxicity) were compared between Group 1 and 2. Further, the impact of learning period (1998–2000) and protocol period (2001–2008) on the fulfillment of PAs for dose prescription and clinical outcome was analyzed.
Results. In the PA+ group there were 77 (34%) and in the PA- group 148 (66%) patients. In the PA- group, CTVHR D90 < 85 Gy was prescribed in 82 patients, D2cm3 bladder > 90 Gy was prescribed in 80 patients and D2cm3 Rectum > 70 Gy in 60 patients. Fulfillment of the PA for dose prescription improved from 4% in the learning period to 48% in the protocol period. The five-year event-free interval was 64% in the learning period and 84% in the protocol period (p = 0.008).
Conclusion. Fulfillment of all PAs for dose prescription is challenging – especially in patients with more advanced tumors. However, with growing experience fulfillment of PA for dose prescription can be significantly increased (learning and protocol period). Such increase in fulfilling PA for dose prescription is followed by a significant improvement in clinical outcome.
The gold standard in the treatment of locally advanced cervix cancer is radio-chemotherapy and brachytherapy [Citation1]. Image-guided adaptive brachytherapy (IGABT) is an advanced method which is increasingly implemented in clinical practice [Citation2,Citation3]. By imaging of the applicator and tumor/target volume and the surrounding organs at risk (OAR), IGABT offers the possibility of dose optimization based on the individual high risk (HR) clinical target volumes (CTV) and OARs [Citation4–7]. First clinical results indicate a reduction in local recurrence and side effects in comparison to standard point A based brachytherapy [Citation8–11]. Recently, dose response relationships were described in IGABT for the target volume and OARs (bladder and rectum) based on the mono- institutional Vienna cohort and also based on the large multi-center cohort RetroEMBRACE [Citation12–15]. The observational prospective multicenter study “EMBRACE” (European study on MRI guided BRAchytherapy in locally advanced CErvix cancer) was initiated to provide further evidence on correlations between radiation dose and clinical findings. Based on such dose response relationships, in-house planning aims (PAs) and constraints were established in various centers for CTVHR and intermediate risk CTV (CTVIR) and OARs. Defined PAs for dose prescription are also projected for the upcoming interventional study “EMBRACE II” study. Moreover, the methodology of PAs and dose prescription has been developed within the upcoming ICRU report on cervix cancer brachytherapy (ICRU report 88) along the lines of ICRU report 83. In clinical practice of IGABT in cervix cancer, fulfillment of all PAs for dose prescription is challenging and often leads to compromises in dose distributions either for the dose prescription for target volumes or for the OARs [Citation10].
Therefore, the aim of our study was: 1) to evaluate the fulfillment of defined PAs (representing correlations to favorable outcome [Citation12–15] in IGABT) for dose prescription in a consecutive patient cohort; 2) to assess whether a growing level of experience with IGABT has impact on the fulfillment of such PAs for dose prescription; and 3) if an increase in fulfillment of such PAs for dose prescription translates into improved clinical outcome.
Material and methods
Patients and work up
All patients with cervical cancer FIGO IA-IVA treated with curative intent at the Department of Radiation Oncology at the Medical University of Vienna 1998–2008 were included in this study. Pre-treatment evaluation included patient history, complete physical and gynecological examination, routine laboratory tests, abdominal computed tomography (CT) or whole body positron emission tomography (PET)-CT and magnetic resonance imaging (MRI) scan of the pelvis.
Follow-up investigations were performed every three months in the first two years after treatment, then every six months in the next two years and then yearly thereafter and included gynecological examination and regular MRI scans of the pelvis and thoracic/abdominal CT scans.
Treatment
The treatment characteristics were already described in previous publications [Citation8,Citation9]. In short, patients were treated by external beam radiotherapy (EBRT), concomitant chemotherapy (Cisplatin 40 mg/m2 of body surface per week for 5 weeks) and IGABT. EBRT was performed initially by four-field box technique and then progressively replaced by IMRT. The prescribed dose was 45 Gy in 1.8 Gy per fraction and was increased to 50.4 Gy if concomitant chemotherapy was not delivered. At the end or after EBRT, MRI-based IGABT was performed using tandem ring applicators +/− interstitial needles, and/or combination of tandem and vaginal cylinders +/− interstitial needles. MRI-based IGABT was started between 1998 and 2000 (‘learning period’) with minor dose optimization, dose escalation and very limited use of interstitial needles whereas from 2001 to 2008 (‘protocol period’) with increasing experience more advanced dose optimization and a higher number of interstitial needles were applied.
For treatment planning and DVH-analysis all doses were calculated as EQD2 sum of all BT fractions and EBRT fractions (α/β = 10 Gy for target volume and α/β = 3 Gy for OARs). The following PAs were applied retrospectively for the evaluation of IGABT in the present study: CTVHR D90 ≥ 85 Gy, D2cm3 Rectum < 70 Gy, D2cm3 Bladder < 90 Gy.
Study design, endpoints and statistical analysis
For this study, patients were stratified into two treatment groups: Group 1, PA+: all dose constraints fulfilled for dose prescription (CTVHR D90 ≥ 85 Gy, D2cm3 Rectum < 70 Gy and D2cm3 Bladder < 90 Gy); Group 2, PA-: one or more dose constraints not fulfilled for dose prescription (CTVHR D90 < 85 Gy or D2cm3 Rectum ≥ 70 Gy or D2 2cm3 Bladder ≥ 90 Gy). PAs were defined based on evidence from Vienna experience [Citation12–14] and from multicenter experience [15 and unpublished evaluations from EMBRACE] and as projected as PAs for EMBRACE II.
Furthermore, patients were stratified according to the treatment period (learning period 1998–2000 vs. protocol period 2001–2008). Differences in patient, tumor and treatment characteristics between the groups were assessed using an unpaired t-test. To reflect the full scope of treatment failure any grade 3 or 4 toxicity and local failure were scored as an event and the event-free interval was calculated and compared using the Kaplan-Meier method and log-rank test.
For time to event variables the durations were calculated from the date of biopsy until the date of event or last follow-up. Local failure was defined as any residual and progressive disease or tumor relapse in uterine cervix, uterine corpus, vagina, parametrium or pelvic wall. Late toxicity was defined as persistence of side effects after 90 days after finished treatment (EBRT+ BT). It was assessed according to Late Effects in Normal Tissue-Subjective, Objective, Management and Analytic LENT SOMA scale [Citation16], from grade 0 (without side effects) to grade 4 (severe). This study focused on severe – grade 3 or 4 – toxicity.
All statistical analyses were performed using SPSS v. 20 and a p-value of < 0.05 was considered significant.
Results
Patients and outcome
Our patient cohort consisted of 225 consecutive patients with locally advanced cervical cancer. The mean age at treatment was 58 years (range 26–92). Eighty-five percent of patients had squamous cell carcinoma. The International Federation of Obstetrics and Gynecology stage (FIGO) distribution in our cohort was; stage I: 25 (11%), stage II 137 (61%), stage III 53 (24%) and stage IV 10 (4%) patients. In PA+ group were 77 (34%) and in PA- 148 (66%) patients. Details on patient characteristics are given in .
Table I. Patients’ cohort description and comparison of PA+ (group 1) and PA- (group 2).
Comparison of planning aim groups
In the PA+ group CTVHR D90 > 85 Gy, D2cm3 bladder < 90 Gy and D2cm3 Rectum < 70 Gy were fulfilled in all patients. In the PA- group, CTVHR D90 < 85 Gy was present in 82 patients, D2cm3 bladder > 90 Gy was present in 80 patients and D2cm3 Rectum > 70 Gy in 60 patients resulting in 222 violations in 148 patients. The mean prescribed dose to CTVHR D90, D2cm3 Rectum and D2cm3 Bladder was significantly different in PA+ and PA- group (98 Gy, 60 Gy, 79 Gy vs. 85 Gy, 67 Gy, 96 Gy). Local failure was observed in 5/77 patients (6.5%) in the PA+ and 23/148 patients (15.5%) in the PA- group; of the 23 PA- patients with local failure, 20 had CTVHR D90 < 85 Gy, 11 had D2cm3 Bladder > 90 Gy and seven had D2cm3 Rectum > 70 Gy. Grade 4 rectal toxicity was observed in two cases in PA+ (both D2cm3 Rectum < 70 Gy) and one case in PA- group (D2cm3 Rectum > 70 Gy). Grade 3 rectal toxicity was observed in no case in PA+ and in four cases in PA- group. Grade 4 bladder toxicity was observed in no case in PA+ and in two cases in PA- group. Grade 3 bladder toxicity was observed in four cases in PA+ and in one case in PA- group. The probability of five-year event-free interval (for local failure and G4 toxicity) in PA+ and PA- group was 90% and 79.1%, respectively. The probability of five-year event-free interval (for local failure and G3 + G4-toxicity) in PA+ and PA- group was 83.9% and 75.6%, respectively (p = 0.086). There were several significantly confounding factors between the PA+ and PA- groups. Most relevant, more advanced tumors and a significantly larger mean CTVHR volume were found in the PA- than in the PA+ group (48 cm3 vs. 34 cm3, p = 0.001) ().
Comparison of time periods
During 1998–2000 (learning period) 71 (33%) patients were treated and 154 (68%) patients from 2001 to 2008 (protocol period). Patient characteristics of the different time periods are shown in . All PAs were fulfilled in 4% of the patients in first period and in 48% in the second period. Mean CTVHR was comparable in both groups. Mean CTVHR D90 was significantly lower in first period compared to second period, (81Gy vs. 92.6 Gy, p < 0.001). There was also a significant reduction in mean D2cm3 for bladder in the second period (86.1 Gy versus 98.8 Gy, p < 0.001), but there was no significant difference in D2cm3 rectum. The five-year local failure-free interval was 72.8% and 91% in first and second period, respectively (p = 0.008). Five-year event-free interval (local failure and G4-toxicity) was 71% in first period and 88.7% in second period (p = 0.007). Five-year event-free interval (local failure and G3 + G4 toxicity) was 64% in first period and 84% in second period (p = 0.008) ().
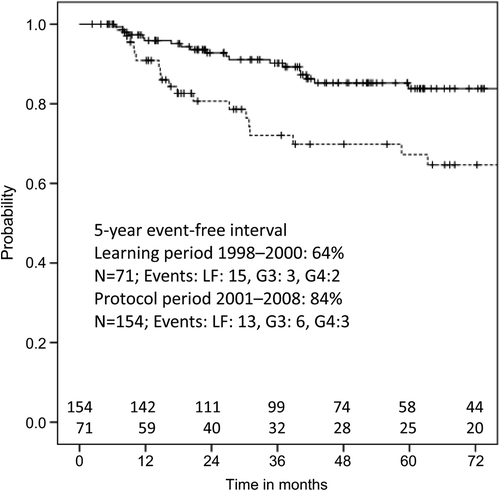
Table II. Comparison of treatment periods 1998–2000 and 2001–2008.
Discussion
In this study we investigated the impact of PAs and dose prescription on the clinical outcome of IGABT. For this purpose local failure and severe – including potentially life-threatening – side effects were summarized within one category. This approach was chosen to mirror the full burden of treatment failure as for the individual patient likely both – a local failure and a severe side effect – will have a tremendous impact on her further life, death or major impairment in quality of life. In consequence, such an approach may be valuable to indicate the full clinical benefit of a given treatment. The possibility of delivering very focused high doses in a short time with brachytherapy is on the one hand the backbone of its high effectiveness, but it can also potentially lead to severe side effects and local failures as a result of inadequate dose distribution [Citation17–19]. Volumetric imaging for brachytherapy treatment planning is therefore an indispensable tool to improve, adapt and individualize the dose distribution [Citation2,Citation3,Citation10]. Evidence-based PAs/dose constraints for clinically relevant endpoints are becoming subsequently more and more a necessity in brachytherapy treatment planning. For IGABT of cervical cancer Dimopoulos et al. showed that if the CTVHR D90 is ≥ 87 Gy a local tumor control > 90% is achievable. Furthermore, the D2cm3 for rectum and bladder were identified as predictive toxicity parameters with an ED10 of 73 Gy for rectum and ED10 of 101 Gy for urinary bladder [Citation12,Citation20]. Based on these findings, PAs for dose prescription have been developed for IGABT at Vienna Medical University with lowering dose values for OARs, in particular for the bladder. The current study shows, however, that fulfillment of all PAs is overall challenging. Even in the protocol period in more than half of the patients, one or more PAs were not fulfilled for the final prescription of treatment. Main reasons might be found in the still missing prospective use of the nowadays proposed PAs, but also in high CTVHR volumes and unfavorable pelvic topography. Tanderup et al. already described the strong influence of the CTVHR volume on the brachytherapy dose distribution [Citation10]. The comparison of the PA+ and PA- group confirms that in prognostic unfavorable patients fulfillment of PAs for dose prescription is in general more difficult. Therefore, a direct comparison of clinical outcome is problematic between these two groups. In addition, due to the highly heterogeneous prescribed doses, the clinical outcome of the PA- group needs to be taken with caution. The results of the PA+ group, in contrast, may be considered as potential benchmark, however, in a selected and rather favorable cohort where less patients with large tumors and unfavorable topography are represented. From a clinical point of view these results show that for patients where all PAs were fulfilled for dose prescription, there is a remaining risk of 10% to develop a major treatment failure event in terms of local recurrence or G4 morbidity. Further research will be necessary to clarify to which extent improvements in dose distribution in patients represented by the PA- group in our study will be followed by improvements in clinical outcome.
In agreement with reports from prostate cancer brachytherapy [Citation21–24], increasing experience seems to have a strong impact on the fulfillment of PAs for dose prescription in IGABT of cervical cancer. In the learning period 1998–2000 only 4% of the patients fulfilled all dose aims for dose prescription, whereas in the protocol period from 2001 to 2008 this number could be increased to 48%. Reasons for this overall dosimetric improvement in patients with comparable CTVHR volumes (learning period: mean CTVHR volume: 42cm3 vs. protocol period: mean CTVHR volume: 44cm3) may be found in the increasing systematic use of interstitial needles and more advanced dose optimization techniques [Citation25,Citation26]. Further dose escalation for the CTVHR and dose constraints for OARs could be established only after some years of experience. The more targeted dose distribution was followed by a significant improvement of the five-year event-free interval by 20% (). The more systematic use of chemotherapy in the protocol period may also contribute to these findings, however, the absolute benefit of concomitant chemotherapy was shown to be mean 6% in a recent meta-analysis [Citation27]. This implies that within the treatment concept of (MR-) IGABT there seems to be certain variability in local outcome and morbidity based on doses applied to the CTVHR and to OARs, the degree of dose optimization and likely other clinical factors which have to be further elaborated. Recent comparisons of conventional 2D (x-ray, point A based) brachytherapy and 3D (MRI, CT) IGABT in cervical cancer showed correspondingly even larger improvements, e.g. 16–35% increase in local tumor control and overall survival in mono-institutional cohorts compared to historical treatment [Citation28,Citation29] and 50% reduction of side effects by IGABT in a prospective comparative study [Citation30].
Interestingly, the improvements in clinical outcome in our study were mainly driven by the increase in local tumor control – severe toxicity did not seem to be substantially influenced by following the PAs for dose prescription (in contrast to low to moderate grade toxicity which was not subject of the current study). However, the incidence of grade 3 + 4 toxicity was overall low. In consequence, the results of the study suggest, that – if reasonably achievable – the highest priority in treatment planning should be aimed at reaching the PAs for dose prescription for the CTVHR.
In conclusion, fulfillment of all PAs for dose prescription is challenging – especially in patients with more advanced tumors. However, with growing experience fulfillment of PAs for dose prescription can be significantly increased (learning and protocol period). Such increase in fulfilling PAs for dose prescription is followed by a significant improvement in clinical outcome.
Declaration of interest: The Department of Radiation Oncology at the Medical University of Vienna receives/received financial and/or equipment support for research and educational purposes from Nucletron, an Elekta company, Varian Medical Systems, Inc., and Isodose Control B.V.
References
- Hanna TP, Shafiq J, Delaney GP, Barton MB. The population benefit of radiotherapy for cervical cancer: Local control and survival estimates for optimally utilized radiotherapy and chemoradiation. Radiother Oncol 2015;114:389–94.
- Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2004;74: 235–45.
- Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77.
- Palmqvist T, Dybdahl Wanderas A, Langeland Marthinsen AB, Sundset M, Langdal I, Danielsen S, et al. Dosimetric evaluation of manually and inversely optimized treatment planning for high dose rate brachytherapy of cervical cancer. Acta Oncol 2014;53:1012–8.
- Haack S, Kallehauge JF, Jespersen SN, Lindegaard JC, Tanderup K, Pedersen EM. Correction of diffusion-weighted magnetic resonance imaging for brachytherapy of locally advanced cervical cancer. Acta Oncol 2014;53:1073–8.
- Schmid MP, Fidarova E, Pötter R, Petric P, Bauer V, Woehs V et al. Magnetic resonance imaging for assessment of parametrial tumour spread and regression patterns in adaptive cervix cancer radiotherapy. Acta Oncol 2013;52:1384–90
- Hegazy N, Pötter R, Kirisits C, Berger D, Federico M, Sturdza A, et al. High-risk clinical target volume delineation in CT-guided cervical cancer brachytherapy: Impact of information from FIGO stage with or without systematic inclusion of 3D documentation of clinical gynecological examination. Acta Oncol 2013;52:1345–52.
- Pötter R, Dimopoulos J, Georg P, Lang S, Waldhäusl C, Wachter-Gerstner N. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83: 148–55.
- Pötter R, Georg P, Dimopoulos J, Grimm M, Berger D, Nesvacil N. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100: 116–23.
- Tanderup K, Nielsen SK, Nyvang GB, Pedersen EM, Røhl L, Aagaard T, et al. From point A to the sculpted pear: MR image guidance significantly improves tumor dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol 2010;94:173–80.
- Nomden CN, de Leeuw AA, Roesink JM, Tersteeg RJ, Moerland MA, Witteveen PO, et al. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: A single institution experience. Radiother Oncol 2013;107:69–74.
- Dimopoulos JC, Lang S, Kirisits C, Fidarova EF, Berger D, Georg P, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2009;75: 56–63.
- Georg P, Kirisits C, Goldner G, Dörr W, Hammer J, Pötzi R, et al. Correlation of dose-volume parameters, endoscopic and clinical rectal side effects in cervix cancer patients treated with definitive radiotherapy including MRI-based brachytherapy. Radiother Oncol 2009;91:173–80.
- Georg P, Pötter R, Georg D, Lang S, Dimopoulos JC, Sturdza AE, et al. Dose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image-guided adaptive cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys 2012;82:653–7.
- Tanderup K, Fokdal L, Sturdza AE, Mazeron R, Kirisits C, Lindegaard JC, et al. Dose and volume response for local control in locally advanced cervical cancer treated with EBRT combined with MRI-guided adaptive brachytherapy. Int J Radiat Oncol Biol Phys 2014;90:S91–2.
- Routledge JA, Burns MP, Swindell R, Khoo VS, West CM, Davidson SE, et al. Evaluation of the LENT-SOMA scales for the prospective assessment of treatment morbidity in cervical carcinoma. Int J Radiat Oncol Biol Phys 2003;56: 502–10.
- Kirisits C, Pötter R, Lang S, Dimopoulos J, Wachter-Gerstner N, Georg D. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2005;62:901–11.
- Mazeron R, Castelnau-Marchand P, Dumas I, del Campo ER, Kom LK, Martinetti F, et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother Oncol 2015; 114:257–63.
- Schmid MP, Kirisits C, Nesvacil N, Dimopoulos JC, Berger D, Pötter R. Local recurrences in cervical cancer patients in the setting of image-guided brachytherapy: A comparison of spatial dose distribution within a matched-pair analysis. Radiother Oncol 2011;100:468–72.
- Georg P, Lang S, Dimopoulos JC, Dörr W, Sturdza AE, Berger D, et al. Dose volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2011;79:356–62.
- Liu HW, Malkoske K, Sasaki D, Bews J, Demers A, Nugent Z, et al. The dosimetric quality of brachytherapy implants in patients with small prostate volume depends on the experience of brachytherapy team. Brachytherapy 2010;9:202–7.
- Beaulieu L, Evans DA, Aubin S, Angyalfi S, Husain S, Kay I, et al. Bypassing the learning curve in permanent seed implants using state-of-the-art technology. Int J Radiat Oncol Biol Phys 2007;67:71–7.
- Battermann JJ, van Es CA. The learning curve in prostate seed implantation. B Cancer Radiother 2000;(Suppl):119–22.
- Le Fur E, Malhaire JP, Baverez D, Delage F, Perrouin-Verbe MA, Schlurmann F, et al. Impact of learning curve and technical changes on dosimetry in low-dose brachytherapy in prostate cancer. Strahlenther Oncol 2012;188:1091–5.
- Fokdal L, Tanderup K, Hokland SB, Røhl L, Pedersen EM, Nielsen SK, et al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol 2013;107:63–8
- Kirisits C, Lang S, Dimopoulos J, Berger D, Georg D, Pötter R. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys 2006;65:624–30.
- Vale C, Tierney JF, Stewart LA, Brady M, Dinshaw K, Jakobsen A. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802–12.
- Rijkmans EC, Nout RA, Rutten IH, Ketelaars M, Neelis KJ, Laman MS, et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol 2014;135: 231–8.
- Lindegaard JC, Fokdal LU, Nielsen SK, Juul-Christensen J, Tanderup K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;51:1510–9.
- Charra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol 2012;103:305–13.
