Abstract
Aim: The aim of this study was to contribute to the collected knowledge of prognostic factors in primary breast sarcomas (PBS) to the benefit of possible future prospective studies and therapeutic guidelines. Method: All patients with pathologically verified PBS in the period of 1979–2014 were extracted from a hospital-based database at Aarhus University Hospital. All records were reviewed for patient and tumor characteristics. Primary endpoints were overall survival, disease-free survival (DFS) and disease-specific survival (DSS). Adjustments were made for age, tumor location, surgical strategy, size, histological classification, prior radiation and grade. Prognostic factors were determined by the use of Cox proportional hazard ratio. Results: In total 42 patients were identified. Surgical resection was the main method of treatment. Nineteen (45%) patients were initially selected for lumpectomy, of these 68% needed at least one re-excision to attain wide margins. In total 55% experienced recurrence, loco regional in 43%. Five-years overall survival was 49%, five-year DFS was 48% and five-year DSS was 40%. Significant prognostic factors were size and grade. A trend towards better survival in those with superficial tumors was observed as well as an increased incidence in radiation-induced angiosarcoma (AS) of the breast, however, prognosis was no different from non-radiation-induced AS. Conclusion: Prognostic factors in PBS patients were size and grade with a trend towards better survival in those with superficial tumors. There was no difference in survival between radiation-induced and spontaneous breast sarcomas. High rate of local recurrence suggests the need for aggressive surgical approach or the routine addition of postoperative radiotherapy in those selected for breast conserving surgery (BCS).
Primary breast sarcoma (PBS) is a rare malignant disease accounting for approximately 1% of all breast malignancies and less than 5% of all sarcomas [Citation1–3]. It involves a heterogeneous group of sarcomas, commonest malignant phyllodes tumor and angiosarcoma (AS) and arises from the mammary gland, mammarian soft tissue, subcutis or dermis [Citation2,Citation4–6]. A major etiological factor influencing the development of PBS is prior radiation to the breast [Citation1,Citation4,Citation7]. The diversity and low incidence of PBS have made prospective studies almost impossible and most published articles are limited to case reports and small scale retrospective reviews, and thus diagnostics and appropriate management of PBS remain challenging.
There is a general agreement that surgical treatment with wide margins is the first line treatment in PBS patients [Citation3,Citation7–9]. However, strategies on local treatment approach are not aligned and the benefit of breast conserving surgery (BCS) versus mastectomy as well as the role of radiotherapy in PBS is undetermined and consensus on optimal treatment approach has yet to be reached [Citation1,Citation3,Citation4,Citation6,Citation9].
This is a retrospective study of PBS patients treated in a single institution over a period of 35 years. We have analyzed the cohort in order to investigate the effect of potential tumor- and treatment-related factors on overall survival (OS) and disease-specific survival (DSS). The aim was to contribute to the collected knowledge of prognostic factors in PBS to the benefit of possible future prospective studies and therapeutic guidelines.
Patients and methods
Patient cohort
This retrospective study has been approved by the Danish Health and Medicines Authority (Ref. Number 3-3013-606/1).
The study population constitutes all patients diagnosed with PBS at Aarhus University Hospital in the time period from 1979 to 2014. All PBS patients were referred to and treated in cooperation with the Sarcoma Centre of Aarhus University Hospital, Denmark. The National pathological database was queried from January 1979 to May 2014 to identify all patients diagnosed with PBS at Aarhus University Hospital, covering primarily the county of middle Jutland (Region Midt, population 1.25 millions) but to a larger extent also the whole of Jutland, resulting in 70 cases. The medical records of only 60 cases were available. They were obtained and retrospectively reviewed for demographics, presentation, diagnostic workup, type of operation, pathology and follow-up including recurrence of disease and death. Pathological reports were reviewed to define primary surgical margins as wide, marginal or intralesional according to the principles of Enneking. The original assessment of resections margins may have varied through the period, however, routinely 1–3 sections perpendicular to the closest margin was cut, if the macroscopic distance to the margin was less than 10 mm. For distances greater than 10 mm either no section or one section tangentially to the margin was cut. Likewise 1–3 sections were taken from the profound margin.
All cases of soft tissue sarcomas (STSs) according to the WHO classification were included, along with phyllodes tumors of malignant and borderline variants and dermatofibrosarcoma protuberans. One patient with atypical vascular lesions of the breast was included as the lesion was impossible to distinguish from low-grade radiation-induced AS.
Primary surgery was defined as the first surgical attempt at achieving wide margins excluding any following re-excisions. Final surgery was defined as last attempt at surgical resection with wide margins including any number of re-excisions. Modified mastectomy included removal of the fascia but not musculature. Radical mastectomy included removal of Pectoralis musculature. The group of radical mastectomy also included singular cases of chest wall resection. Mastectomy (radical and modified radical) in this study did not include axillary dissection unless specified.
Patients with metastatic disease to the breast (n = 2), benign lesions (n = 2), primary sarcomas arising outside the breast tissue (n = 2), carcinoma of the breast (n = 4) and unspecified neoplasm or non-sarcoma neoplasm (n = 4) were excluded as well as patients with a follow-up of less than two years. A total 42 patients were included in this analysis.
Statistics
Primary endpoints of this study were DSS, OS and disease-free survival (DFS). DSS was calculated from the date of diagnosis to time of death attributed to sarcomas or last follow-up. OS was defined as time from date of diagnosis to time of death of any cause or last follow-up. DFS was calculated from the date of diagnosis to the date of pathologically confirmed evidence of relapse or last follow-up. Potential prognostic factors were analyzed by Cox proportional hazard model. Univariate analyses were performed for the following factors: Age at time of diagnosis (divided at the median), grade (I or II vs. III) (as recommended by the WHO the grading system FNCLCC/Trojani was used, prior to 2007 to some extend also Myhre Jensens), size (<5 cm vs. ≥5 cm), radiation-induced (yes vs. no), type of surgery (BCS vs. modified or radical mastectomy), resection margin (wide vs. marginal or intralesional), depth of tumor (superficial vs. deep) and histological subtype [AS vs. phyllodes tumor vs. all others]. Multivariate analysis was performed for the significant factors from the univariate analysis. A p-value of less than 0.05 was considered significant. All analyses were performed by the STATA software version 13.
Results
Patient characteristics and tumor pathology
In total 42 patients, 39 females and males were included in our analysis. Patient and treatment characteristics are summarized in . Fifteen patients had a previous history of cancer, 13 carcinomas of the breast, one pulmonary cancer and one malignant melanoma. Of the 13 patients with previous carcinoma of the breast, 11 (84%) were later diagnosed with AS in the same breast (range 5–10 years, median years: 7). These were all previously irradiated and considered to be radiation-induced.
Table I. Patient and treatment characteristics.
Correct and full histological diagnoses were achieved by means of core biopsy in 14 (33%) cases, 14 (33%) underwent excision biopsy and another 11 (26%) mastectomy before correct diagnoses were attained. Data were missing in three (7%) patients. Six (14%) patients were initially falsely diagnosed with benign lesions that were later revealed to be malignant tumor. These were all ASs, but one. Of the eight phyllodes tumors included in our study, two were initially deemed benign but later transformed into malign tumors.
The two main histological subtypes observed in our study were AS (n = 14, 33%) and phyllodes tumor (n = 8, 19%). All histological subtypes are summarized in . At the time of diagnosis 28 (67%) patients had tumor underneath the fascia separating subcutis from breast tissue.
Table II. Histological classification.
Treatment method and intent
Surgery was performed in 40 (95%) patients with curative treatment intent and in two (5%) patients with palliative treatment intent because of advanced disease. Wide margin after primary surgery was observed in 18 (42%) cases whereas another 16 (38%) patients needed at least one re-excision to attain wide margins. Final wide margin was therefore attained in 34 (81%) patients. Marginal resection was the outcome of final surgery in two (5%) cases and intralesional resection in one (2%) case. In five (12%) cases margin status was not available. Of the 16 cases with marginal or intralesional margin after primary resection, two (12%) had modified radical mastectomy as primary treatment, while the remaining 14 (88%) cases had their primary surgical treatment in the form of BCS.
In total 19 (45%) patients were initially selected for BCS as primary treatment strategy. In this group 68% (n = 13) had at least one re-excision in order to attain wide margin. Of the 19 selected for BCS seven (37%) ended up having modified mastectomy as final treatment. The remaining 12 (63%) had BCS as final surgical treatment with or without re-excision. None of the 10 patients with primary radical mastectomy had pathologically positive margins.
Axillary dissection was performed in four (10%) cases, revealing lymph node involvement in one case of AS.
Postoperative adjuvant therapy was not routinely administered. Only four (10%) patients with undifferentiated pleomorfic sarcoma, fibrosarcoma, phyllodes tumor and AS received adjuvant radiotherapy (Rth). One patient with AS was judged to be at a very high risk of recurrence and also received adjuvant chemotherapy (ifosfamide + doxorubicin in six cycles).
Twenty-three (55%) patients experienced relapse (). Thirteen (57%) of these were treated with curative intent, of which five (38%) had complete response. One died from other causes after four years and the rest are still disease-free with a range of 2–9 years after treatment. Surgery was the preferred treatment modality for recurrence regardless of treatment intent and 17 (74%) had either surgery or surgery and additional adjuvant radio- or chemotherapy. Four patients were only treated with chemotherapy and two patients with chemotherapy plus radiation.
Clinical outcome
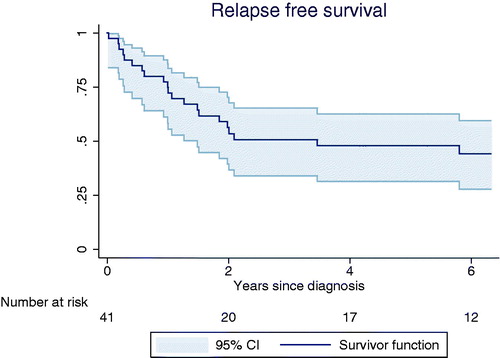
Follow-up data was complete for all patients. Median follow-up for all patients was 4.2 (0.1–27) years. For those alive and with a cut-off of two years follow-up the median follow-up time was 10 (2–27) years. The median time to recurrence was 1.9 years with 66% (n = 16) of relapses occurring within the first year after diagnosis. Another 21% (n= 5) relapsed during the second year after diagnosis and only two (13%) patients relapsed more than three years after diagnosis ().
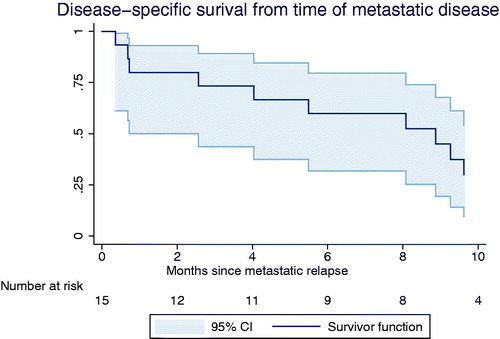
Local recurrence was seen in 10 (24%) cases; two of those had both local recurrence and metastatic disease. Metastasis alone was seen in 13 (31%) cases. Organs affected were lungs (n = 14), bones (n = 7), mediastinum/thorax wall (n = 5), liver (n = 4), lymph nodes (n = 3), contra lateral breast (n = 2), adrenal gland (n = 2), spleen (n = 2). Orbit, abdomen, ovaries or axillary metastasis were seen in one case each. In patients with metastatic relapse DSS was low. They had a median survival of eight moths (95% CI 1; 9.6) and after two years less than 25% were still alive ().
We found that patients treated with BCS had a significantly lower rate of relapse when compared to modified or radical mastectomy (p = 0.038, HR: 0.17, 95% CI 0.03; 0.91). When looking at BCS versus all other forms we found that surgical treatment strategy did not correlate with risk of metastatic or local recurrence (p = 0.75) nor did it influence OS or DSS.
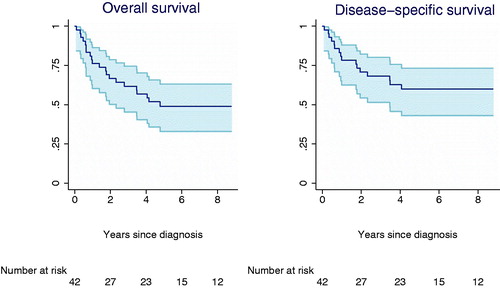
Of the 42 patients, 17 (40%) died from sarcoma and five (12%) of other or unknown causes. The five-year OS was 49% (95% CI 0.33; 0.63) and the five-year DSS was 40% (95% CI 0.27; 0.57) (). We found that DSS was significantly influenced by tumor grade (p = 0.046, HR: 8.011, 95% CI 1.04; 61.1) for patients with grade 3 tumors compared to patients with grade 1 and 2 tumors (). DSS was also significantly influenced by tumor size (p = 0.001, HR: 7.60, 95% CI 2.57; 22.5) and depth of tumor (p = 0.040, HR: 4.73, 95% CI 1.07; 20.91) with a trend towards better prognosis in those with superficial tumor. This association, however, was lost in multivariate analysis. Pathological subtype did not influence OS or DSS. The same was true for age and the extent of final resection (wide, marginal, intralesional). Local recurrence-free survival was 73% (95% CI 0.53; 0.86) and metastatic-free survival 66% (95% CI 0.47; 0.79). Due to the importance of statistic validity we could not perform any uni- or multivariate analysis on these.
Table III. Univariate analysis of factors influencing disease-specific survival.
We found an increase in patients diagnosed with PBS over the last three decades (). The increase was primarily noted in AS of the breast, as 86% (n = 12) of all ASs in this study were diagnosed within the last 10 years (2003–2014) accounting for more than 50% of all PBS diagnosed in this period of time.
Eighty-six percent (n = 12) of ASs in this study were considered to be radiation-induced, but we found that prior radiation to the breast was not a significant prognostic factor for OS or DSS in PBS patients.
Discussion
The rarity, heterogeneous pathology, and the unavailability of prospective studies on PBS underline the importance of studies like this one. Conclusions from such studies, however, must be considered with caution given the limitations inherent to retrospective analysis of a small number of patients.
Our study showed an increase in the number of patients diagnosed with radiation-induced AS of the breast in the last three decades probably as a result of the increased use of breast conserving therapy and better survival rates of patients with breast cancer [Citation10,Citation11], a fact that warrants careful long-term follow-up and swift diagnostic intervention once suspicion of radiation-induced AS is raised.
Our patient population resembles that of patients diagnosed with STS in other sites in the same institute and period of time regarding median age, stage at diagnosis, tumor depth, tumor size and grade [Citation12–14].
Compared to other PBS studies, our cohort has a median age at time of diagnosis of 57 which is higher than the majority of publications reporting a median age of 50 years or less [Citation1,Citation2,Citation4,Citation5,Citation15,Citation16]. High-grade tumors represented 65% of our materials compared to 30–50% in other studies [Citation1,Citation2,Citation7,Citation9]. This difference may be caused by difference in selection criteria between studies, with some studies excluding both radiation-induced sarcomas and phyllodes tumors.
The few studies on PBS that report on stage at time of diagnosis resemble our cohort with a rate of disseminated disease at time of diagnosis between 3% and 10% [Citation1,Citation2,Citation5,Citation15].
The percentage of patients achieving wide margin after primary surgery was rather low (42%) compared to the 95% wide margins achieved for patients with STS in other sites in the same institute [Citation12] as well as to patients with carcinoma of the breast (76%) [Citation17]. Even after final surgery only 81% ended up with wide margin which is relatively low.
Surprisingly, we did not find wide margin to be a prognostic factor, but this could be due to the small number of patients and should not be considered a justification for accepting less than wide margin. We consider wide margin to indisputably remain the goal of surgery with curative intent in agreement with best surgical standards [Citation4,Citation16,Citation18,Citation19].
In this study patients treated with BCS had significantly lower relapse rate than those subjected to modified or radical mastectomy. However, size is a significant prognostic factor and a strong confounder and patients subjected to modified or radical mastectomy will most definitely have more advanced disease making this finding strongly biased. Moreover, 88% of the patients who needed re-excision in order to achieve wide margin was initially treated with BCS, and 33% of all patients initially selected for BCS needed mastectomy to attain wide margin. On this ground BCS cannot be recommended as standard therapy in PBS patients.
Recurrence in both STS and PBS patients is most often seen within five years, a pattern also recognizable in our cohort (), however, both local and distant recurrence were seen as late as 20 years after presentation in this as well as other reports [Citation5,Citation14,Citation16,Citation20,Citation21].
The rate of local recurrence in this cohort (22%) is higher than local recurrence rates observed in STS patients treated in the same institution (16%) [Citation12], which may relate to the relatively low rates of final wide margin. We further found a lower median survival in metastatic PBS patients (8 months) compared to a median survival for metastatic STS of at least 12 months [Citation22]. Collectively, this may be taken as evidence to suggest the need for an aggressive local therapy with mastectomy and perhaps the routine use of radiotherapy.
This approach was shown to be effective in other PBS studies [Citation6,Citation16] and the use of radiotherapy in STS in general is a well established procedure in selected patients [Citation20,Citation23]. Unfortunately, the role of radiotherapy in PBS patients cannot be determined from this study as only four patients received adjuvant postoperative radiotherapy.
We found a DFS of 48%. In comparison most studies report DFS rates around 40–50%, however, DFS as low as 28% has been reported [Citation1,Citation7,Citation15,Citation16,Citation21]. This might reflect methodological and statistical limitations but more than that, it could also be owing to the many different pathological subgroups herein. Especially the fraction of AS and tumors larger than 5 cm in a study cohort might impact survival outcome [Citation1,Citation2,Citation5,Citation11].
Our five-year DSS of 40% naturally also depicts this heterogeneity with the major subtype being AS, however, no patients were lost to follow-up, recurrence was pathologically confirmed and cause of death was known in all cases but one, all of which makes our DSS rate very solid.
Many studies have investigated the influence of tumor size and grade on survival of patients with PBS [Citation1,Citation2,Citation4,Citation5,Citation9,Citation16,Citation21] with no uniform conclusion. It seems, however, that increasing size and especially size above 5 cm carries a worse prognosis [Citation2,Citation5,Citation6,Citation9,Citation21] and the fraction of patients with a tumor larger than 5 cm might influence the impact of size on survival rates.
In contrast to most other primary malignancies, a review of the literature on PBS shows no clear relationship between tumor grade and outcome [Citation2,Citation5,Citation6,Citation19,Citation24]. However, given the strong relationship between grade and STS in general [Citation6,Citation14] the failure to demonstrate a clear association in many studies may be a consequence of the small number of patients in these studies. Our study highlighted that size and grade is indeed significant prognostic factors in patients with PBS, and from this study it seems that PBSs have the same prognostic factors as common STSs, such as size, depth and pathological grade [Citation3,Citation9,Citation14,Citation21]. This can be used to support the notion of a similar treatment approach as that of STS patients.
Normally PBS is considered a superficial tumor type [Citation25] we, however, found that tumor location in the breast and underneath the subcutaneous fascia is a prognostic factor and might affect DSS. This relation, however, was lost in multivariate analysis, but even so, depth of the tumor is a known prognostic factor in STS in general and it would be important to include tumor depth in future studies as another plausible prognostic factor.
We found no association between histological subtype and DSS, but the small number of patients with each subtype makes any conclusions impossible. Frequently retrospective studies of PBS exclude phyllodes tumors of any kind from the cohort on the grounds of different histological origin [Citation2,Citation6,Citation9], however, we found no evidence of prognostic difference in DSS or OS between malignant or borderline phyllodes tumors and other breast sarcomas. This is inconsistent with the findings of other studies [Citation3,Citation15,Citation21], however, a larger scale study than this one might reveal differences and no treatment-related conclusions can be drawn. Grading malignant phyllodes tumors can be challenging, sometimes even impossible, and in five of eight cases of malignant phyllodes tumors in our study obtaining grade was not possible. Furthermore, two tumors originally deemed benign with time transformed into malignant phenotypes. These two factors illustrate the complexity of phyllodes tumor. Based on our data we cannot draw any conclusion regarding grading of phyllodes tumor and survival but we underline the need for regular follow-up regardless of grade or malignancy.
The same complexity applies to ASs in our cohort. They are known to have a large potential of recurrence even in low-grade tumors because of their infiltrative nature [Citation21]. Moreover, normally deemed benign atypical vascular lesions have the potential to transform into malignant tumors making AS a challenging subtype with regards to diagnostics and treatment. Our findings highlight this as five of six with a false benign diagnosis were ASs, correlating to a fraction of 36% of all ASs. Whether these lesions were initially benign and later transformed or whether they were in fact wrongly diagnosed is not possible to determine.
We found no evidence that radiation-induced AS has a worse prognosis compared to non-radiation-induced tumors. This is in contrast to other reports on radiation-induced sarcomas in the breast and elsewhere [Citation1,Citation10] and it is difficult to get a clear answer on this question unless one has a large amount of material with similar distribution of major known prognostic factors.
Lastly, the atypical pattern of metastasis in orbit, abdomen, ovaries and axillary lymph nodes is different from metastatic patterns seen in STS patients and may suggest that computed tomography of the thorax, abdomen and pelvic needs to be part of the control program for these patients.
Conclusion
We found that prognosis of PBS is dependent on tumor size and grade with a trend towards better survival in superficial tumors located in the skin and subcutis. There seems to be an increasing incidence of radiotherapy-induced AS of the breast but our results showed that its prognosis is no different than other spontaneous breast sarcomas.
Surgery with wide margins remain the cornerstone of treatment in patients with PBS, but the high rate of local recurrence seen in this group of patients may suggest the need for aggressive surgery or the routine addition of postoperative radiotherapy in those selected for BCS.
References
- Pencavel T, Allan CP, Thomas JM, Hayes AJ. Treatment for breast sarcoma: A large, single-centre series. Eur J Surg Oncol 2011;37:703–8.
- Barrow BJ, Janjan NA, Gutman H, Benjamin RS, Allen P, Romsdahl MM, et al. Role of radiotherapy in sarcoma of the breast – a retrospective review of the M.D. Anderson experience. Radiother Oncol 1999;52:173–8.
- Al-Benna S, Poggemann K, Steinau HU, Steinstraesser L. Diagnosis and management of primary breast sarcoma. Breast Cancer Res Treat 2010;122:619–26.
- Shabahang M, Franceschi D, Sundaram M, Castillo MH, Moffat FL, Frank DS, et al. Surgical management of primary breast sarcoma. Am Surg 2002;68:673–7; discussion 7.
- Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: Clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer 2004;91:237–41.
- Pencavel TD, Hayes A. Breast sarcoma – a review of diagnosis and management. Int J Surg 2009;7:20–3.
- Bousquet G, Confavreux C, Magne N, de Lara CT, Poortmans P, Senkus E, et al. Outcome and prognostic factors in breast sarcoma: A multicenter study from the rare cancer network. Radiother Oncol 2007;85:355–61.
- McGregor GI, Knowling MA, Este FA. Sarcoma and cystosarcoma phyllodes tumors of the breast – a retrospective review of 58 cases. Am J Surg 1994;167:477–80.
- Fields RC, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Treatment and outcomes of patients with primary breast sarcoma. Am J Surg 2008;196:559–61.
- Sheth GR, Cranmer LD, Smith BD, Grasso-Lebeau L, Lang JE. Radiation-induced sarcoma of the breast: A systematic review. Oncologist 2012;17:405–18.
- Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005;11:241–7.
- Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, Baerentzen S, Pedersen AB, Keller J. Prevalence and prognostic impact of comorbidity in soft tissue sarcoma: A population-based cohort study. Acta Oncol 2014;53:1188–96.
- Maretty-Nielsen K. Prognostic factors in soft tissue sarcoma. Dan Med J 2014;61:B4957.
- Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, Jorgensen PH, Hansen BH, Baerentzen S, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: A cohort study of 922 consecutive patients. Acta Orthopaed 2014;85:323–32.
- Confavreux C, Lurkin A, Mitton N, Blondet R, Saba C, Ranchere D, et al. Sarcomas and malignant phyllodes tumours of the breast – a retrospective study. Eur J Cancer 2006;42:2715–21.
- McGowan TS, Cummings BJ, O’Sullivan B, Catton CN, Miller N, Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys 2000;46:383–90.
- Wilke LG, Czechura T, Wang C, Lapin B, Liederbach E, Winchester DP, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: A report from the National Cancer Data Base, 2004–2010. JAMA Surg 2014;149:1296–305.
- Blanchard DK, Reynolds CA, Grant CS, Donohue JH. Primary nonphylloides breast sarcomas. Am J Surg 2003;186:359–61.
- Smola MG, Ratschek M, Amann W, Samonigg H, Mayer R. The impact of resection margins in the treatment of primary sarcomas of the breast. A clinicopathological study of 8 cases with review of literature. Eur J Surg Oncol 1993;19:61–9.
- Tseng WW, Amini B, Madewell JE. Follow-up of the soft tissue sarcoma patient. J Surg Oncol 2015;111:641–5.
- Zelek L, Llombart-Cussac A, Terrier P, Pivot X, Guinebretiere JM, Le Pechoux C, et al. Prognostic factors in primary breast sarcomas: A series of patients with long-term follow-up. J Clin Oncol 2003;21:2583–8.
- Chen C, Borker R, Ewing J, Tseng WY, Hackshaw MD, Saravanan S, et al. Epidemiology, treatment patterns, and outcomes of metastatic soft tissue sarcoma in a community-based oncology network. Sarcoma 2014;2014:145764.
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. New Engl J Med 2005;353:701–11.
- Gutman H, Pollock RE, Ross MI, Benjamin RS, Johnston DA, Janjan NA, et al. Sarcoma of the breast: Implications for extent of therapy. The M. D. Anderson experience. Surgery 1994;116:505–9.
- Voutsadakis IA, Zaman K, Leyvraz S. Breast sarcomas: Current and future perspectives. Breast 2011;20:199–204.