Abstract
Background: Multiple myeloma (MM) patients who have progressed following treatment with both bortezomib and lenalidomide have a poor prognosis. In this late stage, other effective alternatives are limited, and patients in Sweden are often left with best supportive care. Pomalidomide is a new anti-angiogenic and immunomodulatory drug for the treatment of MM. Our objective was to evaluate the cost effectiveness of pomalidomide as an add-on to best supportive care in patients with relapsed and refractory MM in Sweden.
Material and methods: We developed a health-economic discrete event simulation model of a patient’s course through stable disease and progressive disease, until death. It estimates life expectancy, quality-adjusted life years (QALYs) and costs from a societal perspective. Effectiveness data and utilities were taken from the MM-003 trial comparing pomalidomide plus low-dose dexamethasone with high-dose dexamethasone (HIDEX). Cost data were taken from official Swedish price lists, government sources and literature.
Results: The model estimates that, if a patient is treated with HIDEX, life expectancy is 1.12 years and the total cost is SEK 179 976 (€19 100), mainly indirect costs. With pomalidomide plus low-dose dexamethasone, life expectancy is 2.33 years, with a total cost of SEK 767 064 (€81 500), mainly in drug and indirect costs. Compared to HIDEX, pomalidomide treatment gives a QALY gain of 0.7351 and an incremental cost of SEK 587 088 (€62 400) consisting of increased drug costs (59%), incremental indirect costs (33%) and other healthcare costs (8%). The incremental cost-effectiveness ratio is SEK 798 613 (€84 900) per QALY gained.
Conclusion: In a model of late-stage MM patients with a poor prognosis in the Swedish setting, pomalidomide is associated with a relatively high incremental cost per QALY gained. This model was accepted by the national Swedish reimbursement authority TLV, and pomalidomide was granted reimbursement in Sweden.
Multiple myeloma (MM) is an incurable relapsing haematological disease that requires lifelong, regular care. The incidence of MM in Sweden is approximately 600 cases per year and is slightly more frequent among men [Citation1]. With the mean age at diagnosis of 76 years, most patients with MM are elderly (>65 years), but there are also cases among younger individuals in their 30s and above. In some cases, the disease progression is slow, and patients can be asymptotic for several years. The relative one- and three-year survival rate between 2008 and 2011 was 85.2% and 65.4% for men and 83.0% and 61.1% for women, respectively [Citation2].
Currently, the first-line treatments for most patients in Sweden are triplet combinations including bortezomib (Velcade®; Janssen-Cilag International NV, Belgium) or thalidomide (Thalidomide Celgene®; Celgene International, Boudry, Switzerland) with corticosteroids and alkylating chemotherapy, with bortezomib being preferred in the majority of these combinations. Lenalidomide (Revlimid®; Celgene International, Boudry, Switzerland) is a major drug at second-line and is typically used at relapse. Earlier treatment combinations are often reused if the initial response lasted for a substantial period. Eventually, however, most patients become refractory to these treatments and consequently survival is very poor. Patients who have progressed following treatment with both bortezomib and lenalidomide have a poor prognosis, with a median overall survival of 3–9 months [Citation3,Citation4]. At this late stage effective alternatives are limited, and patients in Sweden are often left with best supportive care.
Pomalidomide (Imnovid®; Celgene International, Boudry, Switzerland) is a new anti-angiogenic and immunomodulatory drug for treatment of MM. In August 2013 it was approved by the European Commission in combination with dexamethasone for treatment of adult patients with relapsed MM refractory to both lenalidomide and bortezomib [Citation5]. According to the results from an open label Phase III clinical trial, with 302 patients randomised to pomalidomide plus low-dose dexamethasone (POMdex) versus 153 patients to high-dose dexamethasone (HIDEX), in patients who have received two prior therapies, POMdex significantly prolongs progression-free survival (PFS) and overall survival [Citation6]. In the trial, at 10 months follow-up, the median PFS with POMdex was 4.0 months versus 1.9 months with HIDEX. Median overall survival was significantly greater in the POMdex group than in the HIDEX group – 12.7 months versus 8.1 months, respectively. Similar results in another group of late-stage MM patients have been presented from a recent study by Kumar et al. [Citation3].
The objective of this work was to evaluate the cost effectiveness of pomalidomide as an add-on to best supportive care in patients with relapsed and refractory MM in Sweden. A cost-utility model was developed to estimate life expectancy weighted into quality-adjusted life years (QALYs), healthcare costs and indirect costs associated with POMdex compared with HIDEX.
Material and methods
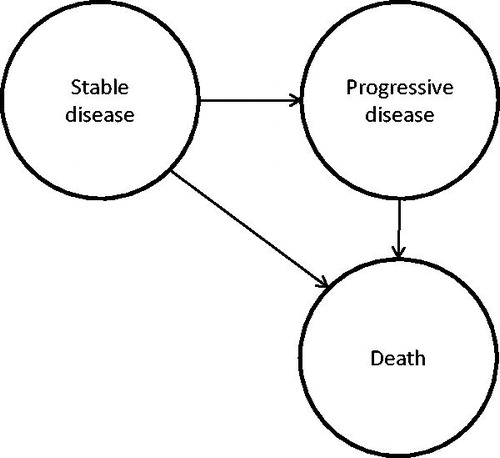
We developed a simple discrete event simulation model with three health states (). The patient starts in stable disease, and then proceeds either to progressive disease and subsequently to death or directly to death. Upon entry, patients are refractory to both bortezomib and lenalidomide, alone or in combination, and refractory to the last treatment. The model was populated with mean values to estimate a patient’s expected costs, LYs and QALYs over the patient’s remaining lifetime.
The model has been used for the reimbursement application for pomalidomide in Sweden. The Swedish reimbursement authority Tandvårds- och läkemedelsförmånverket (TLV) reviewed the application and approved it in June 2014 [Citation7]. This decision represents a validation of the model and its applicability to the Swedish healthcare system.
Time in stable disease and progressive disease
The model parameters that describe the disease course were populated from the MM-003 trial, a comparison between pomalidomide plus POMdex and HIDEX [Citation6]. In the trial, HIDEX patients who experienced disease progression were allowed to cross over to pomalidomide or pomalidomide plus low-dose dexamethasone treatment. Without adjustment, the survival after progression in the HIDEX arm might be overestimated, and therefore, we used estimates of time spent in progressive disease adjusted for treatment cross over [Citation4]. The resulting parameter values are presented in .
Table I. Disease progression parameters.
These estimates depend on parametric survival curves fitted to patient-level data on PFS and overall survival from the MM-003 trial [Citation4]. We tried exponential, Weibull, extreme value, log-normal and log-logistic distributions. The best fit was obtained using log-normal survival curves for overall survival and log-logistic curves for PFS. The log-normal survival distribution has a relatively long tail, that is, long-term survival is relatively high in a small proportion of patients, but this is in line with clinical experience. Nonetheless, while the use of parametric curves allows extrapolation beyond the duration of the trial, a degree of uncertainty is invariably associated with the tails of these curves. Statistical goodness-of-fit measures for parametric survival curves only apply to the time period of the clinical trial. For alternative models of PFS and overall survival beyond observed follow-up, we derived estimates of the time spent in the health states from the MM-003 Kaplan-Meier survival curves. As the number of patients remaining under follow-up gradually decreases, we attached a parametric tail at the point where less than 15 patients remained under observation. The tail represented a constant risk of death/progression, taken from the observed Kaplan-Meier data up to that point. The HIDEX survival curves were in this case adjusted for treatment switching using a risk ratio. Being based on data from a shorter time period, the long-term survival using these constant risk curves diminishes more rapidly. The resulting parameters were mean time in progression-free 6.0 months in POMdex and 2.7 months in HIDEX, and mean survival in progressive disease is 10.3 months in POMdex and 4.7 months in HIDEX, and these were used in a sensitivity analysis.
Although it seems reasonable to adjust for treatment switching, there are a variety of methods that can be used for this adjustment. We estimated costs and QALYs without adjusting for treatment switching to understand the sensitivity of the model to cross over adjustment. In this analysis, we accrued costs for the mean 4.5 months of pomalidomide treatment observed within the trial (data on file) to account for the patients who switched to pomalidomide in the HIDEX arm. Here, we used a within-trial analysis based solely upon the Kaplan-Meier curves, with truncated tails (i.e. no survival beyond the point where <15 patients were under follow-up).
Costs
Drug costs were taken from the Swedish Pharmacopeia as pharmacy retail price (AUP) [Citation8]. In the model, dexamethasone was taken as 40 mg/day during four days (low dose), or 12 days (high dose), per 28-day treatment cycle. The resulting costs per 28-day treatment cycle were SEK (Swedish Krona) 496 (€52.70 based on exchange rate SEK 1 = €9.41, 9 September 2015) for low-dose, and SEK 1490 (€158.34) for high dose, based on a cost of SEK 1241 for a 100 × 4 mg pack. Pomalidomide was taken in 28-day cycles, 4 mg/day on Days 1–21. The cost of pomalidomide was SEK 76 213 (€8099.15) per 28-day treatment cycle, based on a 21 × 4 mg pack for SEK 76 213.
The costs for resource use in connection to laboratory tests and monitoring were taken from a health-economic evaluation of lenalidomide in Sweden [Citation9]. The annual cost per patient was estimated to be SEK 18 362 (€1951.32) before progression, and SEK 40 569 (€4311.26) after progression (Supplementary Table I, available online at http://www.informahealthcare.com).
An approximate calculation of the costs for adverse events was based on the cost per event in the evaluation of lenalidomide [Citation9], applied to POMdex and HIDEX adverse event rates in the MM-003 trial [Citation6]. This resulted in costs of SEK 11 690 (€1242.30) in the POMdex arm, and SEK 8180 (€869.29) in the HIDEX arm (Supplementary Table II, available online at http://www.informahealthcare.com). In two sensitivity analyses, we investigated the influence of these estimates by doubling the cost in one treatment arm and zeroing the cost in the other arm ().
A societal perspective is usually taken in cost-effectiveness analyses in Sweden. Indirect costs are accrued as net production minus consumption during the patient’s remaining life [Citation10] (). An adjustment is made for the proportion of patients who work – the indirect cost at a given age is taken as the value of production adjusted for the proportion of patients who work, minus the value of consumption. To accommodate recent changes in the guidelines for economic evaluation of drugs for reimbursement applications to TLV [Citation11], we conducted a sensitivity analysis including productivity costs but without consumption for added LYs. We also conducted a sensitivity analysis without indirect costs to facilitate comparisons to other analyses using a healthcare perspective, although it should be emphasised that economic analyses are not easily transferrable between countries.
Table II. Annual production and consumption per person.
Utilities
The main outcome in the model was LYs weighted into QALYs using utilities estimated from EQ-5D data collected in the MM-003 trial [Citation12], with a time trade-off based tariff [Citation13] and disutilities associated with adverse events according to the Catalogue of EQ-5D scores for the UK [Citation14]. Average utilities by disease progression and treatment arm were derived using a generalised estimating equations regression approach – 0.65 before and 0.62 after progression for POMdex, and 0.61 before and 0.59 after progression for HIDEX.
Treatment discontinuation
In the clinical trial MM-003, treatment was discontinued at disease progression [Citation6]. However, in real clinical practice, treatment may continue if progression is slow and there are few disease symptoms. The MM-003 trial defined progression as a 25% increase or an increase of at least 5 g/l of the M component. However, a patient can satisfy this criterion but still be symptom-free (e.g. if the M component increases slowly enough). Here, treatment is likely to continue despite the trial’s progression criterion being met. Also if no other treatment options are available, treatment may continue simply to avoid the situation where a patient is left without further treatment. Treatment may also be discontinued before disease progression due to side effects or lack of response. In our base case analysis, we assume that treatment continues precisely to the point of progression (or death, whichever comes first). In a sensitivity analysis, we try to accommodate a real-world treatment discontinuation by assuming that 10% of the patients are treated also beyond progression until death. Additional costs but no additional effectiveness (survival benefit) are assumed in this analysis. In another sensitivity analysis, we also assumed that another 10% of the patients discontinue treatment early. Based on the MM-003 trial, these patients were estimated to spend on average 0.68 months in stable disease without pomalidomide treatment. The parameter values in this sensitivity analysis were used in the base case in the TLV application.
Suite of analyses and model evaluation
In our main analysis, the patients starting in the model are assumed to be 64 years of age, based on the MM-003 trial population [Citation6], and we assume 25% of them are working. This assumed figure represents that a portion of the patients will work part of the time before disease progression.
To explore the direction and magnitude of changes in parameter values and assumptions, we carried out a set of deterministic sensitivity analyses that affect how survival is modelled, adjustment for treatment switching, age, the proportion who work, unit costs of adverse events, and adjustment of the model to reflect a real-world setting. This also included a sensitivity analysis with older patients, with a mean age of 70 years, to reflect that the treated population might be older than the trial population. In addition, we carried out an analysis using a healthcare perspective to facilitate comparisons to other countries where such a perspective would be taken. We also used a modified societal perspective where indirect costs did not include consumption during gained LYs, according to Swedish guidelines [Citation11].
We also carried out a stochastic sensitivity analysis of the base case with regards to time spent in the health states and age. This is similar to a probabilistic sensitivity analysis, but it only varies a few of the parameters. It served to roughly estimate the overall uncertainty of the results of our main analysis. The mean time in stable disease and the mean time in progressive disease, in each treatment arm, were sampled from the survival distributions that were fitted to the MM-003 trial data in the adjustment for treatment cross over [Citation4], using the variance and covariance matrix via the Cholesky decomposition method. Age was sampled from an assumed normal distribution with a mean (SD) of 64 (12) years, based on the MM-003 trial population [Citation6]. All other model parameters were held constant.
Costs, LYs and QALYs were discounted using a 3% rate (0% in a sensitivity analysis). Costs are presented as year 2015 SEK (€1 ≈ SEK 9.41). Cost estimates from previous years were converted to year 2015 SEK using the consumer price index [Citation15].
Results
The model analyses a 64-year-old patient, previously treated with two regimens including bortezomib and lenalidomide, alone or in combination, and refractory to the last regimen. Treated with POMdex, the patient has a total cost of SEK 767 064 (€81 516), mainly consisting of pomalidomide costs and indirect costs (). If treated with HIDEX, the total cost is SEK 179 976 (€19 126), two thirds of which are indirect costs. Due to better survival with POMdex compared to HIDEX, the POMdex patient gains two months in stable disease and 12.5 months in progressive disease compared to HIDEX (life expectancy 2.33, and 1.12 years, respectively), which translates into a QALY gain of 0.7351. The incremental cost associated with POMdex is SEK 587 088 (€62 390) consisting of increased drug costs (59%), incremental indirect costs (33%) (where consumption is only slightly offset by gained production) and other healthcare costs (8%). The incremental cost-effectiveness ratio (ICER) is SEK 798 613 (€84 869) per QALY gained.
Table III. Incremental cost-effectiveness analysis.
Table IV. The main analysis and the deterministic sensitivity analyses [costs in Swedish Krona (SEK)].
Deterministic sensitivity analyses
The results from the deterministic sensitivity analyses are presented in . Changes in the assumptions of extrapolation of survival beyond trial and adjustment for treatment switch had the highest impact on the results. Using the Kaplan-Meier based survival curves, and thereby assuming shorter survival after progression, POMdex is associated with lower life expectancy gain and lower QALY gain. In both arms, indirect and monitoring costs are lower than in the base case, but drug costs increase in the POMdex arm, and therefore incremental costs are approximately the same as in the base case, and the ICER becomes SEK 1.2 million (€130 000) per QALY.
When we do not adjust for treatment switching, the HIDEX arm accrues pomalidomide costs. This reduces the incremental cost associated with POMdex to SEK 26 378 (€2803) compared to SEK 587 088 (€62 390) in the base case. The QALY gain becomes 0.1069, and the ICER is estimated to be SEK 246 666 (€26 213) per QALY.
When we use the model to estimate a real-world setting in which 10% of the patients are treated beyond progression, the ICER increases to SEK 1.0 million (€110 000) per QALY. The ICER reduction is negligible when we also assume that 10% of the patients discontinue their treatment early.
Our analyses of the patients show that age and the proportion who work influence indirect costs. In 60-year-old patients, of whom 50% work, the ICER is SEK 611 042 (€64 935) per QALY gained compared to SEK 798 613 (€84 869) in the base case. This reduction is approximately equal parts due to the lower age and the increased production. In 70-year-old patients, of whom none work, the ICER is SEK 802 919 (€85 326) per QALY – similar to the base case. Our variations of the unit costs of adverse events have little influence; the ICER ranges between SEK 771 707 and 825 518 (€82 009–87 728).
In the sensitivity analysis without indirect costs for consumption, total costs on the POMdex arm are halved compared to the base case. The HIDEX arm is estimated to have a negative cost, as low drug costs are more than offset by the value of production. This results in a low overall incremental cost (SEK 388 943, €41 333) and an ICER of SEK 529 076 (€56 225) per QALY gained.
To facilitate comparison to other countries, a healthcare perspective analysis was also performed. Without indirect costs, but otherwise identical to the main analysis, the ICER is estimated to be SEK 533 382 (€56 682) per QALY gained. However, it should be acknowledged that it is difficult to transfer economic results from one country to another.
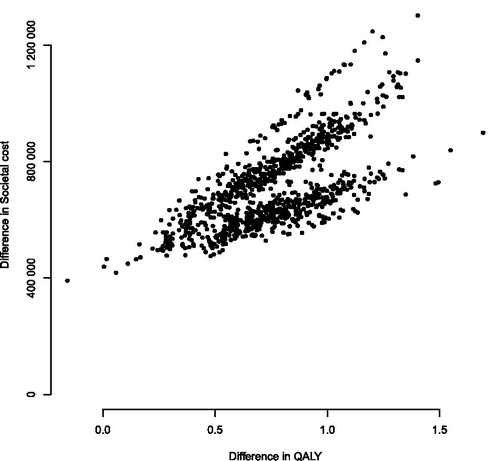
Stochastic sensitivity analysis
Our stochastic sensitivity analysis resampled the time spent in each health state and the age of the patient, under the assumptions of the base case scenario. The variation in the cost-effectiveness plane (i.e. incremental cost and incremental QALY plotted against each other) is shown in . The bands in the figure are related to the age intervals in the indirect cost data. The incremental cost associated with POMdex was estimated to be SEK 560 000 [95% confidence interval (CI) SEK 387 000–792 000) (€59 500; €41 100–84 200), and the QALY gain 0.71 (0.23–1.21). The resulting ICER was SEK 862 000 (€91 600) per QALY (95% CI SEK 641 000–1 007 000; €68 100–107 000).
Discussion
To study the cost effectiveness of pomalidomide and low-dose dexamethasone compared with HIDEX in patients with relapsed and refractory MM in Sweden, we developed a health-economic model of a patient’s course through stable disease and progressive disease, until death. The model estimated life expectancy, QALYs and costs from a Swedish societal perspective. The model was populated with efficacy data and utilities from the MM-003 trial comparing pomalidomide plus low-dose dexamethasone to HIDEX, and cost data from official Swedish price lists, government sources and literature. Patients in the comparator arm of the MM-003 trial were allowed to switch to pomalidomide after progression, and therefore, we adjusted the effectiveness estimates to reflect a situation where patients did not switch.
In the base case, with treatment lasting until disease progression, which is according to indication, the model estimated that pomalidomide and low-dose dexamethasone was associated with incremental costs and incremental QALYs compared to HIDEX, corresponding to an ICER of SEK 798 613 (€84 900) per QALY gained. We conducted sensitivity analyses to identify which parameters influenced results. The adjustment for treatment switching and the assumptions regarding extrapolation of survival beyond trial had the greatest impact on the ICER. When we did not adjust for treatment switching, but accrued pomalidomide costs to the HIDEX arm according to the drug use in the trial, the ICER became SEK 246 666 (€26 200) per QALY gained. Thus, our adjustment does not appear to overestimate the level of cost effectiveness of pomalidomide. The alternative, less optimistic survival assumption increased the ICER to about SEK 1.2 million (€130 000).
We also wished to analyse a scenario closer to the real-world situation with regards to treatment discontinuation. Therefore, we conducted a sensitivity analysis allowing 10% of patients to be treated beyond disease progression. This resulted in an ICER of SEK 1.0 million (€110 000). Here, additional costs but no additional effectiveness (survival benefit) was assumed. Although there are no formal data on effectiveness post-progression, this scenario analysis is likely to be conservative. A patient may also discontinue pomalidomide treatment early, before progression. When we allowed 10% of patients to discontinue early, the ICER was only very slightly reduced. Therefore, treatment after progression is more influential on the ICER than early treatment discontinuation. Finally, one should mention that treatment discontinuation is not a random behaviour, but a decision under the physician’s control that is influenced by several factors (e.g. available alternatives and how fast the patient progresses).
We suspected that in real treatment practice in Sweden, patients may be older than in the clinical trial on which we based our model. Study populations are always selected, e.g. few centres participate, and young patients with no other treatment options are more likely to travel to participate in the trial. In addition, exclusion criteria may prevent older patients with comorbidities from participating. We therefore investigated the influence of age and the proportion who work. This resulted in ICERs in the range of SEK 611 042 (€64 935) per QALY gained in 60-year-old patients, half of whom worked, to SEK 802 919 (€85 326) per QALY gained among 70-year-old non-working patients. Although pomalidomide has been approved for reimbursement in Sweden, we estimate that it will be relatively less cost effective in older patients. However, according to Swedish legislation, age on its own must not be a factor that determines access to healthcare. Furthermore, age only affects indirect costs in our model, whereas in reality, it should also affect life expectancy. This is a weakness of our model, and extrapolation of our results to populations with a substantial variation in mean age should be avoided.
We used a stochastic sensitivity analysis to study the uncertainty surrounding the ICER estimate in the base case scenario. A 95% CI for the ICER was estimated to be between SEK 0.6 and 1.0 million (€68 100–107 000) per QALY gained. The mean time in stable disease and progressive disease by treatment arm were varied in this analysis as well as age. The CI more or less covers the range that we saw with regards to age above, however, in that analysis we also varied the proportion of patients who work.
Our base case ICER and the range of ICERs in the above sensitivity analyses ought to be acceptable in Sweden given the severity of the condition. The TLV did approve reimbursement of pomalidomide in June 2014 [Citation7]. The application to the TLV was based on the model presented here but with different parameter settings although resulting in reasonably similar ICERs. The TLV’s review of the application and subsequent approval represents a form of validation of the model. In the application, the base case analysis was similar to our current real-world sensitivity analysis, but the patients were slightly younger, a higher proportion worked (as indicated in ), and we assumed 10% would discontinue treatment early. The present work uses slightly different assumptions and parameter settings for more elaborated real-world adjustments of treatment discontinuation, patient age and proportion who work. Furthermore, it discounts future costs and QALYs and uses year 2015 costs. In any case, TLVs approval indicates that the ICERs are acceptable in Sweden.
In the MM-003 trial, patients were in the sixth-line of treatment on average. Here, we presume two or more prior treatment lines – potentially an earlier positioning. It is difficult to say what implications this has on the interpretation of the results.
Costs of palliative care have not been included in our analysis. Such costs would appear on both sides of the comparison, with the same magnitude assuming the same duration of palliative care during the last six months prior to death. Furthermore, because they would appear very close in time, discounting would make little difference. Thus, these costs would cancel each other out in the incremental analysis and therefore have no influence on the incremental cost per QALY. At the absolute level, however, the exclusion of these costs does have an impact.
We have not costed concomitant medication that might be used in these advanced MM patients. For POMdex, growth factors like G-CSF and EPO are sometimes used, especially when initiating the therapy. These drugs are expensive and might affect the analysis. However as several of these drugs are available as less expensive biosimilars, we excluded concomitant medication from the analysis.
Conclusion
In a model of late-stage MM patients with a poor prognosis in a Swedish setting, pomalidomide is associated with a relatively high incremental cost per QALY gained. This model was accepted by the national Swedish reimbursement authority TLV, and pomalidomide has been granted reimbursement in Sweden by TLV.
Declaration of interest
Ulf Persson is, and Sixten Borg was, an employee of IHE. Dawn Lee is and Jamie Elvidge was, an employee of BresMed. This manuscript and the cost-effectiveness analyses forming the basis for this work were sponsored by Celgene, Kista, Sweden through IHE and BresMed. Hareth Nahi and Markus Hansson declare no conflicts of interest.
Supplementary material available online
Supplementary_Tables__29.5__15.docx
Download MS Word (23.1 KB)References
- Diagnosgruppen för plasmacellsjukdomar. Myelom utredning och behandling, Nationella riktlinjer fastställda 2010 Nov 15, [Updated 2013 Jan 31].
- Ghatnekar O, Alvegard T, Conradi N, Lenhoff S, Mellqvist UH, Persson U, et al. Direct hospital resource utilization and costs of treating patients with multiple myeloma in Southwest Sweden: A 5-year retrospective analysis. Clin Ther 2008;30:1704–13.
- Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 2012;26:149–57.
- Morgan G, Palumbo A, Dhanasiri S, Lee D, Weisel K, Facon T, et al. Overall survival of relapsed and refractory multiple myeloma patients after adjusting for crossover in the MM-003 trial for pomalidomide plus low-dose dexamethasone. Br J Haematol 2015;168:820–3.
- Celgene International Sàrl. Press release: Oral anti-cancer therapy pomalidomide Celgene now approved by European Commission as treatment for patients with relapsed/refractory multiple myeloma – a rare form of blood cancer, 2013 Aug 9. Celgene International Sàrl, Boudry, Switzerland, 2013.
- Miguel JS, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol 2013;14:1055–66.
- Tandvårds- och läkemedelsförmånsverket (TLV). Beslut 4538/2013. 2014.
- Farmaceutiska Specialiteter i Sverige [Internet]. 2013. [cited 2013 Oct 1]. Available from: http://www.fass.se.
- Celgene. REVLIMID (lenalidomide) – Health economic report. Celgene AB, 2007 Aug 24.
- Ekman M. Consumption and production by age in Sweden: Basic facts and health economic implications. In: Ekman M. Studies in health economics: Modelling and data analysis of costs and survival Dissertation for the degree of Doctor of Philosophy at the Stockholm School of Economics. EFI, Stockholm, Sweden. 2002.
- Tandvårds- och läkemedelsförmånsverket (TLV) Tandvårds- och läkemedelsförmånsverkets allmänna råd. Stockholm 2015.
- Song KW, Dimopoulos MA, Weisel KC, Moreau P, Palumbo A, Belch A, et al. Health-related quality of life from the MM-003 trial of pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed and/or refractory multiple myeloma. Haematologica 2015;100:e63–7.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800–4.
- Price conversion [Internet]. 2013. [cited 2013 Oct 1]. Available from: http://www.scb.se.