Abstract
Background: Many prostate cancer patients die of other causes, but it remains unknown whether comorbidity interacts synergistically with prostate cancer to increase the mortality rate beyond that explained by the individual risks of comorbidity and prostate cancer.
Methods: A nationwide cohort study of 45 326 Danish prostate cancer patients diagnosed during 1995–2011, each matched to approximately five men from the general population on age and individual comorbidities in the Charlson Comorbidity Index (CCI). We calculated five-year mortality rates and interaction contrasts as a measure of the excess mortality rate explained by synergy between prostate cancer and comorbidity.
Results: Five-year mortality was 46.8% in prostate cancer patients and 25.8% in matched men from the general population. For prostate cancer patients with a CCI score of 2–3, the mortality rate was 250 per 1000 person-years [95% confidence interval (CI): 236, 263], and interaction between comorbidity and prostate cancer accounted for 20% of the total mortality rate (50 deaths per 1000 person-years, 95% CI 35, 65) in the first year following cancer diagnosis. The interaction was mainly present for patients with metastatic disease and those not treated with prostatectomy.
Conclusion: Up to 20% of all deaths among men who had both prostate cancer and comorbidities could be explained by the comorbidity-prostate cancer interaction. The mortality attributable to comorbidity itself and the mortality attributable to the interaction may be reduced by successful treatment of the comorbidity.
Prostate cancer is the second most common cancer among men worldwide, with an estimated 913 000 new cases in 2008 [Citation1]. In Denmark, 4316 incident cases were diagnosed in 2012, making prostate cancer the most common malignancy in men nationally. Its incidence increased by 35% from 2003 to 2012 [Citation2]. At the same time, general five-year survival after a prostate cancer diagnosis increased from 34% in 1998 to 60% in 2009 [Citation3]. Trends in incidence and survival after prostate cancer are similar in many European countries and in the United States [Citation4].
In Denmark, prostate cancer patients without comorbidities have experienced a marked increase in five-year survival (from 51% to 73% between 2000 and 2011), while patients with severe comorbidity, defined as a Charlson Comorbidity Index (CCI) score of ≥3, experienced modest improvement in survival (from 33% to 39% between 2000 and 2011) [Citation5]. Prostate cancer patients with comorbidity thus had a more than two-fold increased risk of dying compared with prostate cancer patients who are otherwise healthy [Citation5]. Similar findings have been reported in other settings [Citation6]. Comorbidity may affect cancer detection, treatment decisions and compliance, surveillance and, ultimately, mortality. Still, many prostate cancer patients die from other conditions.
To our knowledge, no study has compared mortality rates for prostate cancer patients with that of men from the general population and examined whether prostate cancer and specific comorbid conditions interact to increase mortality more than expected by each factor acting alone. We therefore conducted a nationwide study of prostate cancer patients and a cohort of men from the general population, matched on age and specific comorbidities.
Methods
Design and populations
This matched cohort study included all prostate cancer patients diagnosed in Denmark between 1995 and 2011 and a comparison cohort of men without prostate cancer from the general population. Denmark has a tax-financed national healthcare system, which provides free access to hospital and outpatient services. A unique Civil Personal Registration (CPR) number, assigned to each Danish resident at birth or upon immigration, is included in all medical and administrative registries. The CPR number is used in research to ensure accurate individual linkage between national registries [Citation7]
All cancer diagnoses in Denmark are recorded in the Danish Cancer Registry (DCR), which has maintained data on national cancer incidence since 1943 [Citation8]. All men registered in the DCR with an incident prostate cancer during 1995 to 2011 were included in the study cohort of prostate cancer patients. The general population comparison cohort was assembled using the Civil Registration System (CRS), which maintains information on civil and vital status for all Danes since 1968 [Citation9]. We matched up to five men from the general population without prostate cancer to each patient with prostate cancer on age and history of the specific comorbidities included in the CCI [Citation10]. We defined the index date as the date of prostate cancer diagnosis for cases and as the corresponding date of matching for men in the general population cohort. Men from the matched cohort who were diagnosed with an incident prostate cancer during follow-up were censored on their diagnosis date and then included in the prostate cancer cohort. They were then matched with a general population sample drawn on the index date.
Comorbid conditions and treatment
All available ICD-8 and ICD-10 discharge diagnoses of the CCI conditions (except for prostate cancer and non-melanoma skin cancer) was extracted from the Danish National Patient Registry (DNPR), which holds all non-psychiatric discharge diagnoses from inpatient hospitalizations since 1977 and all hospital outpatient and specialist visits since 1995 [Citation11]. Using the weights assigned to CCI conditions, we calculated a summary score describing the level of severity of comorbidities (0, 1, 2–3, ≥4).
We also collected information on prostatectomy and androgen deprivation therapy (ADT) from the DNPR. The positive predictive values for pharmacological ADT and surgical ADT have been documented as 93% and 100%, respectively [Citation12]. From the Danish Pathology Registry, we collected information on Gleason Score and D’Amico risk group for patients diagnosed since 2004.
Follow-up
The study outcome was time to death after the index date. Using the CRS, we followed the cohorts from the index date until death, emigration, five years of follow-up, or 31 December 2012, whichever came first. We stratified the follow-up period by the first year and years 2–5 after the index date. As the general population cohort was unlikely to die of prostate cancer, we did not ascertain cause-specific mortality.
Statistical analysis
Members of the two cohorts were characterized according to age group (0–69, 70–74, 75–79, 80–84, and ≥85 years), index year (1995–1999, 2000–2004, and 2005–2011), baseline comorbidity level (none: CCI score of 0; low: CCI score of 1; moderate: CCI score of 2–3; and severe: CCI score ≥4), and the individual comorbidities included in the CCI. The prostate cancer cohort was further stratified by index year categories (1995–1999, 2000–2004, 2005–2011), cancer stage at diagnosis (non-metastatic vs. metastatic), prostatectomy (yes/no), pharmacological ADT (yes/no), bilateral orchiectomy (yes/no), Gleason score (2–6, 7, 8–10) and D’Amico risk group (low-, intermediate-, and high-risk group).
Kaplan-Meier survival analysis was used to estimate mortality risks in the cohorts. Standardized mortality rates were computed using age weights based on the prostate cancer cohort as of the index date. We then performed Cox proportional hazard regression modeling to compute hazard ratios as an estimate of mortality rate ratios (MRRs), based on the standardized rates and adjusting for the CCI score, in overall analyses comparing prostate cancer patients with members of the general population cohort. The matching was dissolved in adjusted analyses and in analyses stratifying the follow-up period. In analyses within strata of comorbidity levels, we adjusted for age (continuous) and index year categories. These analyses also were stratified by non-metastatic cancer (i.e. localized and regional stage) versus metastatic cancer (i.e. distant stage), specific CCI diseases, age groups (0–69 years, 70–79 years, and ≥80 years), and on presence/absence of prostatectomy, pharmacological ADT, and bilateral orchiectomy. For analyses of treatment, we included only patients diagnosed starting in 1996, when the NOMESCO classification of surgical procedures was introduced in Denmark, and we followed patients from the first date of treatment registration. For Gleason score and D’Amico risk group, we only included patients diagnosed between 2004 and 2011. The proportional-hazards assumption was met according to the shape of the log-log plots.
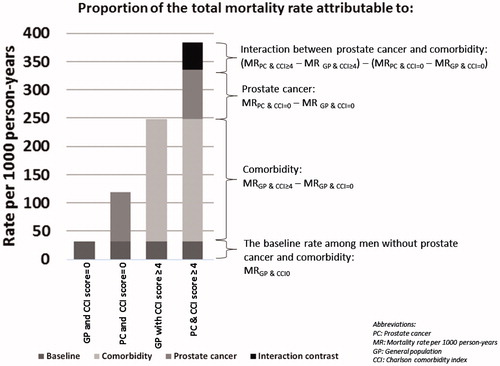
The interaction between prostate cancer and comorbidity on mortality was examined by calculating interaction contrasts, which measure the combined effect of two factors that cannot be explained by combining their individual effects. In the present study, the interaction contrast was calculated on the additive scale by subtracting, from the mortality rate difference among patients with prostate cancer and comorbidity, the sum of mortality rate differences for patients with prostate cancer alone and comorbidity alone. Each mortality rate difference was computed using the same reference group – men without prostate cancer and without comorbidities. The interaction contrast therefore is a measure of the excess or deficit mortality rate above or below what can be expected given the baseline mortality rate among men without prostate cancer and without comorbidity, the effect of prostate cancer on the mortality rate, and the effect of comorbidity on the mortality rate. An example of the calculation of the interaction contrast is presented in .
Figure 1. Example of calculation of the total mortality rate among prostate cancer patients with comorbidity and the proportion of the total mortality rate attributable to the baseline mortality rate among men in the general population without comorbidity, to comorbidity, to prostate cancer, and to interaction between prostate cancer and comorbidity.
Of 45 646 patients, 320 (0.7%) men could not be matched to any member of the general population, and were excluded. The unmatched patients were older (41% were ≥80 years vs. 20% of the matched patients) and had a greater prevalence of a CCI score ≥3 (98% vs. 5.9%).
Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
The study received the required approval from the Danish Data Protection Agency (Record Number: 2011-41-6174).
Results
The study included 45 326 men diagnosed with incident prostate cancer between 1995 and 2011 and 225 106 age- and comorbidity-matched men from the general population. The median age was 72 years (interquartile range: 65, 78 years). As there were fewer than five men from the general population available for matching in the case of several prostate cancer patients, the proportions of matched baseline characteristics were not always exactly equal ().
Table I. Descriptive characteristics of the prostate cancer cohort and the general population comparison cohort, Denmark, 1995–2011.
First year of follow-up
As presented in and , 13.1% [95% confidence interval (CI) 12.8, 13.4] of the prostate cancer patients and 5.03% (95% CI 4.95, 5.13) of men from the general population died during the initial year following the index date, consistent with increased overall one-year mortality among prostate cancer patients (MRR = 2.85, 95% CI 2.76, 2.94).
Figure 2. Cumulative mortality during five years of follow-up in the cohorts of prostate cancer patients and men from the general population matched on age and comorbidities, Denmark 1995–2011.
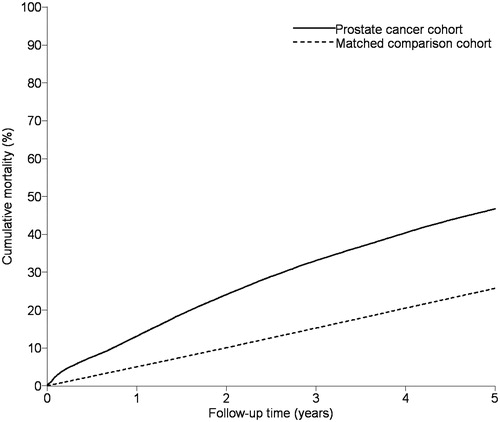
Table II. Charlson Comorbidity Index (CCI) scores, standardized mortality rates, interaction contrasts, and mortality rate ratios in the prostate cancer cohort and matched general population comparison cohort during the first year and years 2–5 of follow-up, Denmark, 1995–2011.
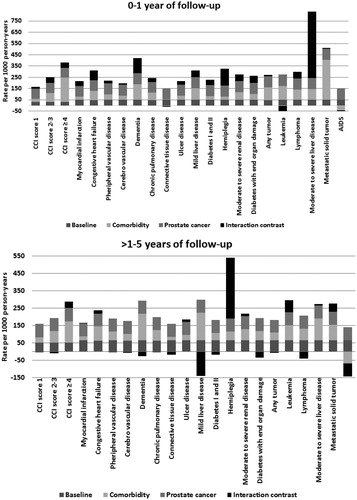
For patients with severe comorbidity (CCI score ≥4), the standardized mortality rate was 383 (95% CI 341, 425) per 1000 person-years (PYs) in prostate cancer patients and 248 (95% CI 232, 263) in men from the general population, corresponding to a mortality rate difference of 135 per 1000 PYs. For men without comorbidity, the corresponding standardized rates per 1000 PYs were 119 (95% CI 115, 124) in patients with prostate cancer and 32 (95% CI 31, 33) for comparison cohort members, corresponding to a rate difference of 87 per 1000 PYs. The interaction contrast is the difference between these rate differences (135 minus 87), corresponds to 48 deaths per 1000 PYs. This represents 13% of the total mortality rate that can be attributed to interaction between prostate cancer and severe comorbidity. Among patients with moderate comorbidity, interaction with prostate cancer represented 20% of the total mortality rate. Most individual CCI diseases interacted with prostate cancer in the first year of follow-up, as shown in .
Figure 3. Proportions of the total standardized mortality rates per 1000 person-years contributed by the baseline rate among men free of comorbidity and prostate cancer, comorbidity, prostate cancer, and interaction between comorbidity and prostate cancer, Denmark, 1995–2011.
MRRs comparing prostate cancer patients with the general population decreased with increasing comorbidity levels, from 3.8 (95% CI 3.6, 4.0) for men without prostate cancer and comorbidity to 1.5 (95% CI 1.3, 1.7) for prostate cancer patients with severe comorbidity. This is consistent with the increasing background mortality associated with greater comorbidity. Stratifying analyses by age groups did not substantially alter the interaction. All levels of comorbidity interacted with metastatic prostate cancer (). In addition, interaction was present for patients not treated with prostatectomy or bilateral orchiectomy. MRRs associated with D’Amico high-risk group was 3.2 (95% CI 2.4, 4.1) compared with the low-risk group (data not shown). The one-year overall MRR comparing prostate cancer patients with the general population comparison cohort decreased slightly from 3.4 (95% CI 3.2, 3.6) for patients diagnosed 1995–1999 to 3.0 (95% CI 2.8, 3.1) for patients diagnosed 2000–2004 and to 2.4 (95% CI 2.3, 2.6) for patients diagnosed 2005–2011.
Table III. Standardized mortality rates and interaction contrasts stratified by age groups, stage at prostate cancer diagnosis, and treatment, Denmark, 1995–2011.
Later years of follow-up
The 2–5 year mortality risks were 38.7% (95% CI 38.2, 39.3) for prostate cancer patients and 21.8% (95% CI 21.6, 22.0) for men from the general population, corresponding to a 2.29-fold increased mortality rate for prostate cancer patients (95% CI 2.25, 2.34). While standardized rates increased with higher comorbidity levels, MRRs comparing prostate cancer patients to matched men from the general population decreased with higher comorbidity scores from 2.86 (95% CI 2.78, 2.94) in prostate cancer patients without comorbidities to 1.5 (95% CI 1.3, 1.7) in patients with high comorbidity levels, consistent with increased mortality risk associated with high comorbidity. Interaction between comorbidity and prostate cancer was present only among patients with severe comorbidity and represented 13% of the total mortality rate (IC = 38, 95% CI -27, 103) per 1000 PYs. This is consistent with the results presented in , showing that interaction had only limited impact on mortality for specific CCI diseases. By age and treatment groups, interaction was present in men younger than 70 years of age and those treated with pharmacological ADT. MRRs associated with D’Amico high-risk group was 2.5 (95% CI 2.2, 2.8) compared with the low-risk group (data not shown).
Discussion
In this large population-based cohort study including prostate cancer patients and matched men from the general population, mortality rates were not dominated by interaction between prostate cancer and comorbidity. However, in the first year after prostate cancer diagnosis, interaction accounted for up to 20% of total mortality rates of prostate cancer patients with comorbidity. In stratified analyses, the interaction was observed primarily for men with metastatic prostate cancer.
Several studies have documented that comorbidity is a strong negative prognostic factor in prostate cancer [Citation5,Citation6], which this study confirms. Severe comorbidity may affect prostate cancer detection by concealing symptoms, limiting potential treatments, and compete as premature causes of death. For example, liver disease, heart disease, and dementia may require adjustment of cancer treatment [Citation13,Citation14]. Radical prostatectomy is offered to men with localized disease and an expected life expectancy of more than 10 years [Citation15], which may explain why no interaction was present among men receiving this treatment. Patients with metastatic prostate cancer, no orchiectomy, and treatment or no treatment with pharmacological ADT had interactions at all levels of comorbidity. Men with a low survival probability associated with older age or severe comorbidity usually undergo conservative watchful waiting or receive ADT. More aggressive treatment with curative intent can be offered to patients without serious competing diseases, which may explain the patterns of interaction we observed [Citation16].
The low and negative interaction contrasts suggest lack of a combined effect of prostate cancer and comorbidity on the mortality rate of prostate cancer patients who survived the first year after diagnosis. It is likely that many of these patients were diagnosed with slowly progressing prostate cancer in the context of opportunistic PSA screening, or had completed prostate cancer treatment with curative intent [Citation17]. In addition, men with localized prostate cancer have been found to have better survival than the general Danish population [Citation18]. A high survival probability may explain some of the low and negative interaction observed in the present study. Also, though still controversial, statins and metformin for hypercholesterolemia and diabetes has been associated with improved survival among prostate cancer patients [Citation19,Citation20].
This study is the first to compare mortality for prostate cancer patients with that of men from the general population, examining interactions with individual comorbidities. Our results resemble those from previous studies of interaction between comorbidity and breast cancer and colorectal cancer. However, a stronger interaction was observed with colorectal cancer than with prostate and breast cancer [Citation21,Citation22]. Survival of colorectal cancer patients is lower than that of breast and prostate cancer patients, and treatment is usually more invasive, which may increase colorectal cancer-related mortality [Citation23]. In addition, a recent study conducted in the United States found that survival from conditions other than cancer was higher for patients with early-stage prostate and breast cancer but similar for patients with colorectal cancer compared with the general population [Citation24].
Our study has several noteworthy strengths. First, prostate cancer diagnoses are almost universally recorded in the DCR [Citation8], and the CRS has nearly complete follow-up data on all study participants. We matched men from the general population to prostate cancer patients, which allowed examination of specific comorbidities and calculation of mortality rates in the prostate cancer cohort and the general population cohort. Second, positive predictive values of comorbidities included in the CCI range from 82% to 100% [Citation25].
Our study also has several limitations. Outpatient diagnoses have been included in the DNPR only since 1995, which could have resulted in under-ascertainment of comorbidities. In addition, we defined presence of comorbidity based on just one primary or secondary diagnosis in the DNPR. We also lacked detailed information about type and duration of prostate cancer therapies.
We also had no information on lifestyle factors and socioeconomic status that potentially could confound the associations under study. However, we believe that these factors had a negligible impact on our study, as we matched men from the general population to prostate cancer patients on individual comorbidities.
In this study up to one in five deaths among men with prostate cancer and comorbidity could be explained by interaction with comorbidity in the first year after diagnosis, driven by patients with metastatic prostate cancer. The mortality attributable to comorbidity itself and the mortality attributable to the interaction may be reduced by successful treatment of the comorbidity.
Acknowledgments
This work was supported by the Danish Agency for Science, Technology and Innovation (Record number: 10-084581), the Danish Cancer Society (R73-A4284-13-S17), the Aarhus University Research Foundation, the Program for Clinical Research Infrastructure established by the Lundbeck and the Novo Nordisk Foundations. The funding sources had no role in design, analysis, or interpretation of the study. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study. The authors declare no conflicts of interest.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917.
- Cancerregisteret. Tal og analyse. 2012. [cited 2014 Sep 10]. Available from: http://www.ssi.dk/~/media/Indhold/DK%20-%20dansk/Sundhedsdata%20og%20it/NSF/Registre/Cancerregisteret/Cancerregisteret%202012.ashx.
- Borre M, Erichsen R, Lund L, Larsen EH, Norgaard M, Jacobsen JB. Survival of prostate cancer patients in Central and Northern Denmark, 1998–2009. Clin Epidemiol 2011;3(Suppl 1):41–6.
- Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079–92.
- Nguyen-Nielsen M, Norgaard M, Jacobsen JB, Borre M, Thomsen RW, Sogaard M. Comorbidity and survival of Danish prostate cancer patients from 2000–2011: A population-based cohort study. Clin Epidemiol 2013;5(Suppl 1):47–55.
- Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med 2013;158:709–17.
- Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 2006;53:441–9.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011;39(7 Suppl):42–5.
- Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–83.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(7 Suppl):30–3.
- Jespersen CG, Borre M, Norgaard M. Validity of the recorded codes of gonadotropin-releasing hormone agonist treatment and orchiectomies in the Danish National Patient Registry. Clin Epidemiol 2012;4:145–9.
- Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: The renal insufficiency and anticancer medications (IRMA) study. Cancer 2007;110:1376–84.
- Raji MA, Kuo YF, Freeman JL, Goodwin JS. Effect of a dementia diagnosis on survival of older patients after a diagnosis of breast, colon, or prostate cancer: Implications for cancer care. Arch Intern Med 2008;168:2033–40.
- Roder MA, Brasso K, Christensen IJ, Johansen J, Langkilde NC, Hvarness H, et al. Changes in preoperative characteristics in patients undergoing radical prostatectomy – a 16-year nationwide analysis. Acta Oncol 2014;53:361–7.
- Klotz L. Active surveillance for prostate cancer: Overview and update. Curr Treat Options Oncol 2013;14:97–108.
- Hjertholm P, Fenger-Gron M, Vestergaard M, Christensen MB, Borre M, Moller H, et al. Variation in general practice prostate-specific antigen testing and prostate cancer outcomes: An ecological study. Int J Cancer 2015;136:435–42.
- Roder MA, Brasso K, Berg KD, Thomsen FB, Gruschy L, Rusch E, et al. Patients undergoing radical prostatectomy have a better survival than the background population. Dan Med J 2013;60:A4612.
- Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol 2010;28:2653–9.
- Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: Reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013;63: 709–16.
- Erichsen R, Horvath-Puho E, Iversen LH, Lash TL, Sorensen HT. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer 2013;109: 2005–13.
- Ording AG, Garne JP, Nystrom PM, Froslev T, Sorensen HT, Lash TL. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis – a Danish Nationwide Matched Cohort Study. PLoS One 2013;8:e76013.
- Lemmens VE, Janssen-Heijnen ML, Verheij CD, Houterman S, Repelaer van Driel OJ, Coebergh JW. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg 2005;92:615–23.
- Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: Other-cause survival and comorbidity prevalence. Am J Epidemiol 2013;178:339–49.
- Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 2011;11:83.
