To the Editor,
Avascular necrosis (AVN) of the femoral head is a rare skeletal disease usually presenting with vague symptoms from the hip or lower pelvis. Risk factors include alcoholism, use of corticosteroids, prior pelvic irradiation and various medical disorders, e.g. sickle cell disease, systemic lupus erythematosus and Gaucher’s disease. The pathophysiology remains unclear, but is believed to include an altered microcirculation and disruption of the blood supply to the femoral head [Citation1,Citation2].
Ewing’s sarcoma (ES) accounts for about 3% of all pediatric and adolescent malignancies and remains the second most common primary bone tumor [Citation3]. Following the introduction of combined modality treatment, the prognosis has dramatically improved with an expected survival of 70–80% for patients presenting with localized disease [Citation4]. Treatment consists of multi-agent chemotherapy, including anthracyclines and alkylating agents with risk of acute and late toxicities, the latter including potential cardiac, gonadal and renal damage [Citation5].
Association between AVN of the femoral head and neoplasms has been described for hematological malignancies both as a presenting symptom of acute leukemia [Citation6,Citation7] and as a complication for treatment of Hodgkin’s lymphoma [Citation8]. For solid tumors sparse and casuistic reports of AVN following treatment of testicular cancer [Citation9] and high-dose chemotherapy with autologous stem cell support for ovarian cancer have been published [Citation10].
To the best of our knowledge we present the first case of AVN of the femoral heads following the treatment course of ES.
Case description
A 28-year-old Caucasian male with no prior medical history was diagnosed in January 2013 with ES located at the right thoracic wall. At the time of diagnosis, positron emission tomography-computed tomography (PET-CT) showed a heterogeneously PET-positive tumor with involvement of costae. There were no signs of metastases with PET-CT imaging, but CT scan showed one suspicious nodule in the left lung that was considered to be a metastasis though biopsy was not taken. An incision biopsy from the primary tumor revealed a classic ES with the characteristic chromosomal translocation t(11;22) and the gene fusion product ESW-FLI-1.
The patient was treated in accordance with the guidelines from the EURO-EWING 99 protocol, consisting of induction chemotherapy with six cycles of VIDE [vincristine 1.5 mg/m2 D1/ifosfamide 3 mg/m2 Days (D) 1–3/doxorubicin 20 mg/m2 D 1–3/etoposide 150 mg/m2 D 1–3] as preoperative treatment. Prednisolone was administered as part of the standard antiemetic regime for each cycle of chemotherapy.
After six cycles of VIDE, a CT scan showed partial response of the tumor in the thoracic wall and total regression of the metastases in the lower left lung.
Four months after diagnosis a wide excision was performed with free resection margins and pathology revealed a tumor necrosis of only 50%. The patient received post-operative radiotherapy to the tumor bed [45 gray (Gy) in 25 daily fractions] as well as whole lung irradiation (18 Gy in 10 daily fractions). Irradiation of the primary site was delivered at M. D. Anderson Cancer Center, Houston, USA, using protons while lung irradiation was given in Denmark using regular photon therapy.
Concomitant with radiotherapy to the tumor bed, post-operative chemotherapy was given in the form of two cycles of ifosfamide single agent by continuous infusion by pump over 14 days. This was followed by six cycles of VAI (vincristine 1.5 mg/m2 D1, actinomycin D 0.75 mg/m2 D 1–2, ifosfamide 3 mg/m2 D 1–2). Following the last cycle of chemotherapy 10 months after the initial diagnosis a CT scan showed complete remission and the patient was clinically asymptomatic. He went into a follow-up protocol with regular CT scans and clinical controls.
Fourteen months after diagnosis new bilateral multiple lung metastases were seen on a follow-up CT scan and the patient started second line chemotherapy with irinotecan and temozolomide (irinotecan 10 mg/m2 D 1–5/temozolomide 100 mg/m2 D 1–5).
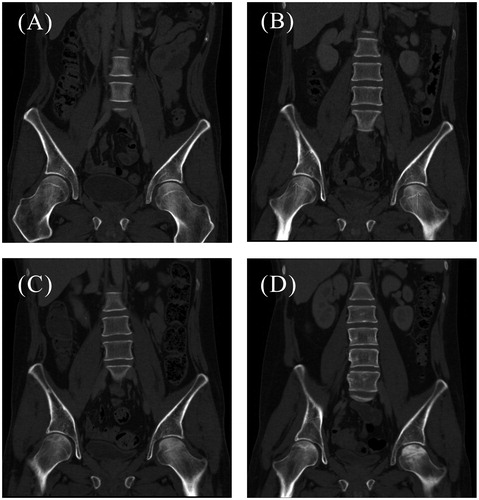
During the follow-up period, approximately 13 months after diagnosis, and before relapse was diagnosed, the patient started complaining of pain in both lower limbs. The pain was described as vague, not localized and sporadic and was initially not investigated further. Later, 16 months after diagnosis, it became constant and localized to both hips, impairing the patient’s ability to walk. The patient’s need for analgesics increased steadily. An investigative MR scan of both hips and the pelvic region revealed bilateral AVN of the femoral heads (). A conservative treatment strategy was chosen so the chemotherapy could continue. The progression of the AVN can be followed (), starting with a normal scan showing no signs of necrosis one year after diagnosis, followed by radiolucency and microfractures at 14 and 17 months, respectively, and at last healing with sclerosing 19 months after diagnosis.
Figure 1. Bilateral osteonecrosis, coronal T1 weighted MRI. 16 months after diagnosis of ES, 2 May 2014. Note that the MR scan fits chronologically between CT scans (B) and (C) in Figure 2.
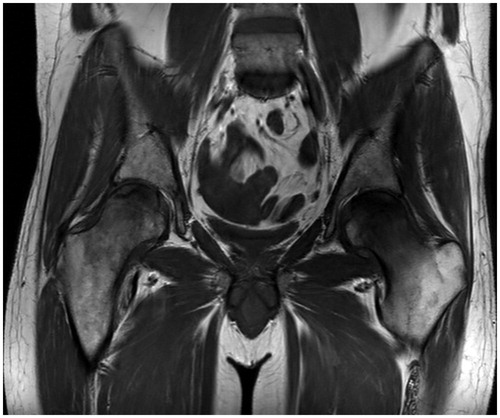
Figure 2. CT scans (A-D) of the patient’s hip region showing the different stages of AVN. (A) Normal femoral heads bilaterally, one year after diagnosis of ES (20 January 2014). (B) Initial signs of necrosis bilaterally (arrows) with radiolucency, 14 months after diagnosis (26 March 2014). (C) Progression of osteonecrosis with radiolucencies and sclerosing lines, 17 months after diagnosis (12 June 2014). (D) Increasing sclerosis of the left femoral head, 19 months after diagnosis (4 August 2014).
Blood samples 17 months after diagnosis showed elevated parathyroid hormone levels of 10.3 pmol/L (1.6–6.9 pmol/L), probably secondary to slightly reduced levels of total 25-hydroxy D-vitamin, 41 nmol/L (50–150 nmol/L).
Unfortunately, the patient progressed on irinotecan and temozolomide as well as on third line chemotherapy with cyclophosphamide (250 mg/m2 D 1–5) and topotecan (0.75 mg/m2 D 1–5). The patient was judged ineligible for further oncological antineoplastic treatment because of impaired kidney function with a declining glomerular filtration rate of 24 ml/min. The necrosis of the femoral heads was managed conservatively with analgesics and supportive care. During the clinical course the pain and walking ability stabilized, so reconstructive surgery was not indicated.
Discussion
This report is the first documented case of AVN following treatment of ES. However, Meneghello et al. [Citation11] described a 32-year-old female with osteosarcoma who developed bilateral AVN of the femoral heads following a chemotherapy regimen not very different from the one administered in this case namely: vincristine, dactinomycin and cyclophosphamide.
For patients with solid tumors, non-traumatic AVN following chemotherapy was first systematically reviewed in the literature in 2001 by Winquist et al. [Citation12]. They found 14 reports describing 39 cases of AVN in patients who had received chemotherapy for solid tumors. The femoral head was the involved bone in 26 of the cases with bilateral involvement in 18. The majority of patients were young males which was also the case when the review was updated in 2008 [Citation13].
This patient therefore, represents the typical demographic profile, being a younger male patient receiving combination chemotherapy. There was no medical history or clinical grounds to suspect other causes of AVN, such as rheumatological diseases, or excessive alcohol consumption. Only standard doses of steroids were used as antiemetics during the chemotherapy, not as maintenance therapy. Therefore excessive use of corticosteroids as a causative factor could also be excluded.
It is possible that the AVN in this case is caused by a combination of low level of vitamin D and the use of metronomic chemotherapy.
The blood samples revealed slightly reduced vitamin D level and slightly elevated parathyroid hormone. Although we do not have other measurements, it is now observed that slight vitamin D insufficiency is a widespread chronic problem in the population of Danish adults [Citation14]. The elevated parathyroid hormone is probably secondary.
Recent studies suggest a coupling between osteogenesis and angiogenesis through the finding of a new subtype of vessel in murine bone [Citation15]. Chronic vitamin D insufficiency could therefore have led to altered microvasculature of the femoral head.
The patient received ifosfamide by pump over 14 days twice while receiving radiotherapy [Citation16]. Chemotherapy given with relative low dose over extended periods of time (metronomic chemotherapy) is known to particularly target endothelial cells and microvasculature, which was probably already defective in our patient.
These two factors may have been contributing to the development of AVN in this particular patient.
In conclusion, we present the first case of bilateral AVN of the femoral heads following standard chemotherapy of ES and emphasize the importance of early detection of this rare complication. Based on theoretical consideration we hypothesize that patients with vitamin D insufficiency and those receiving metronomic chemotherapy may be at particular risk.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amanatullah D, Strauss EJ, Cesare PE. Current management options for osteonecrosis of the femoral head. Am Orthop 2011;40:186–92.
- Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiology 2007;63:16–28.
- Bernstein M, Kovar H, Paulussen, Randall RL, Schuck A, Teot LA, et al. Ewing's sarcoma family of tumors: current management. Oncologist 2006;11:503–19.
- Karosas A. Ewing's sarcoma. Am J Health Syst Pharm 2010;67:1599–605.
- Balamuth N, Womer R. Ewing's sarcoma. Lancet Oncol 2010;11:184–92.
- Gürkan E, Yildiz I, Oçal F. Avascular necrosis of the femoral head as the first manifestation of acute lymphoblastic leukemia. Leuk. Lymphoma 2006;47:365–67.
- Ghosh J, Manjunatha YC, Bakhshi S. Avascular necrosis of femoral head in childhood acute myeloid leukemia: complication of chemotherapy without steroids. Pediatr Blood Cancer 2008;51:308–9.
- Enrici E, Anselmo AP, Donato V, Santoro M, Tombolini V. Avascular osteonecrosis in patients treated for Hodgkin's disease. Eur. J. Haematol. 1998;61:204–9.
- van den Berkmortel F, de Wit R, de Rooy J, DeMulder P. Osteonecrosis in patients with testicular tumours treated with chemotherapy. Neth J Med 2004;62:23–7.
- Bojko P, Hilger RA, Ruehm SG, Dirsch O, Seeber S, Scheulen ME. Femoral head necrosis in three patients with relapsed ovarian cancer receiving high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 2003;31:487–91.
- Meneghello A, Presacco D, Di Maggio C. Aseptic necrosis of the femoral head in cancer patients affected with vincristin/vinblastin induced neuropathy. Radiol Med 1989;77:626–30.
- Winquist E, Bauman G, Balogh J. Nontraumatic osteonecrosis after chemotherapy for testicular cancer: a systematic review. Am J Clin Oncol 2001;24:603–6.
- Shim K, MacKenzie M, Winquist E. Chemotherapy-associated osteonecrosis in cancer patients with solid tumours: a systematic review. Drug Saf 2008;31:359–71.
- Thuesen B, Husemoen L, Fenger M, Jakobsen J, Schwarz P, Toft U, et al. Determinants of vitamin D status in a general population of Danish adults. Bone 2012;50:605–10.
- Kusumbe, AP, Ramasamy, SK, Adams, RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014;507:323–8.
- Zhang Y, Kawedia JD, Myers AL, McIntyre CM, Anderson PM, Kramer M, et al. Physical and chemical stability of high-dose ifosfamide and mesna for prolonged 14-day continuous infusion. J Oncol Pharm Pract 2014;20:51–7.
