Abstract
Background Lung cancer is an increasing problem in the older patient population due to the improvement in life expectation of the Western population. In this study we examine trends in lung cancer incidence and mortality in Denmark from 1980 to 2012 with special focus on the elderly.
Material and methods Lung cancer was defined as ICD-10 codes C33-34. Data derived from the NORDCAN database with comparable data on cancer incidence, mortality, prevalence, and relative survival in the Nordic countries, where the Danish data were delivered from the Danish Cancer Registry and the Danish Cause of Death Registry with follow-up for death or emigration until the end of 2013.
Results In 2012, about 50% of lung cancers were diagnosed among persons aged 70 years or more. For men and women older than 75 years the incidence rates have been increasing and for those aged 80–84 years, the rates have doubled since 1980. Due to the poor survival, similar trends were seen in mortality rates. Over the period, the one-year relative survival rates almost doubled in patients aged 70 years or more, but still only 25% of the patients aged 80–89 years survived their lung cancer for one year.
Conclusion The incidence of lung cancer is closely linked to the pattern of tobacco smoking with the differences between gender and age groups reflecting smoking behavior in birth cohorts. Elderly patients with lung cancer are a heterogeneous group in whom treatment should be offered according to comorbidity and a geriatric assessment.
Lung cancer is one of the most frequent cancer forms in the world and the leading cause of death from cancer worldwide [Citation1]. The worldwide burden and incidence of lung cancer has been rising and the burden is expected to continue to increase well into this century. Lung cancer is common in the elderly with the majority of patients being diagnosed at the age between 65 and 80 years. The patients have a high level of comorbidity. The aging of the population in the Western world have resulted in an increase in the number of older patients diagnosed with lung cancer. The median age of the lung cancer patient in Denmark is 69 years with a prevalence of comorbidity of 50% and a five-year survival rate of less than 15% [Citation2,Citation3].
Smoking is, by far, the most common cause of lung cancer in the general population, being linked directly to 80–90% of all cases of lung cancer [Citation4]. Early in the previous century cigarette smoking was largely confined to men with prevalence peaking among those involved in the First World War with the incidence slowly increasing until the 1950s. Hereafter the incidence more quickly decreased. For women the change in social circumstances and working pattern during World War II caused a rapid increase in tobacco use. Smoking is also a likely explanation for the level of comorbidity among lung cancer patients as, for example, chronic obstructive pulmonary disease (DOPD) and several cardiovascular diseases are linked to smoking [Citation2].
There is a disturbing trend showing that the frequency of patients to undergo treatment for lung cancers decreases with increasing age at diagnosis, even though patients older than 70 years tolerate aggressive multimodality therapy that may enhance their survival. The elderly are frequently excluded from treatment protocols and those selected for treatment are often given gentle or suboptimal doses of conventional chemotherapy [Citation5].
The aim of the present analysis is to describe trends in incidence, mortality, and relative survival of lung cancer in Denmark from 1980 to 2012 focusing on age, comparing persons aged 70 years or more with those aged less than 70 years.
Material and methods
Lung cancer was defined as ICD-10 codes C33-34. A detailed description of the materials and methods appear elsewhere [Citation6]. In brief, data were derived from the NORDCAN database with comparable data on cancer incidence, mortality, prevalence and relative survival in the Nordic countries, where the Danish data are delivered from the Danish Cancer Registry and the Danish Cause of Death Registry with follow-up for death or emigration until the end of 2013. This study focused on the elderly population with age categorized as 0–69, 70–79, 80–89 and 90 + years.
For incidence and mortality, age group specific numbers and rates per 100 000 person years are shown in tables and graphs with calendar periods for time of diagnosis 1978–1982, 1988–1992, 1998–2002, 2003–2007, 2010, 2011 and 2012. Prevalence was defined as the number of cancer patients (including cured patients) with that specific diagnosis still alive and is shown in tables by the end of 1980, 1990, 2000, 2005, 2010, 2011 and 2012.
Sex- and age-specific one- and five- year relative survival proportion ratios were calculated for each of the diagnostic groups for the age groups 0–69, 70–79, 80–89 and 90 + years and for the five-year periods of diagnosis 1968–1972, 1973–1977, …, 2003–2007 and 2008–2012.
Relative survival for a group of cancer patients was calculated as the observed survival (where all causes of death were considered events) divided by the expected survival for a group from the Danish population with the same age and year of birth composition. Actuarial method was used for observed survival and Ederer II method for the expected survival [Citation7]. Relative survival can be interpreted as the survival if the cancer was the only cause of death. For the most recent period, 2008–2012, five-year follow-up for death is not available for all patients and a hybrid method, where we supplement with survival experience from cancer patients diagnosed earlier years, was used. Survival was not calculated for cancer groups with less than five patients (indicated by (-) in tables and blank in the graphs). If all patients died in the follow-up period resulting in zero survival this is indicated as 0 (-) and in cases where the calculation results in a relative survival higher than 100%, the result is shown in tables, but restricted to 100% in graphs.
Results
Incidence
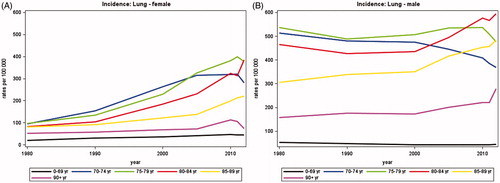
Between 1980 and 2012, the average annual number of newly diagnosed lung cancers remained almost constant in men whereas it increased from 711 to 2193 in women (). In 2012, about 50% of lung cancers were diagnosed among persons aged 70 years or more. The most pronounced increase was observed in persons aged 80–89 years, today accounting for about 17% of all newly diagnosed lung cancers.
Table I. Average annual number of new lung cancers in Denmark, 1980–2012.
Since 1980 the incidence rates of lung cancer among women have increased for all age groups (). Among women aged 70–84, the lung cancer incidence rates have more than tripled since 1980. For women between 70 and 74 years the incidence peaked around 2005 and has been declining since 2010. In the group of women older than 90 years the curve is more uncertain due to small numbers.
Since 1980, the incidence rates of lung cancer decreased for males aged up to 74 years, remained stable for those aged 75–79, and increased for those over 80 years (). It should be noted that for both women and men there was not a straight forward pattern of increasing incidence with increasing age. Persons aged 85 years or more had a lower incidence than those aged 70–84 years, but still higher than those aged less than 70 years.
Mortality
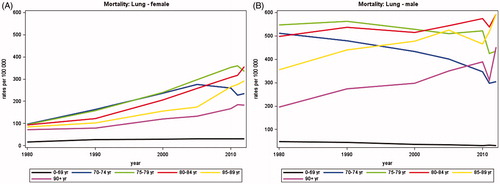
shows that the average annual number of deaths from lung cancer increased in women from 649 in 1980 to 1785 in 2012 while a slight decrease was observed in men from 2103 in 1980 to 1960 in 2012. A total of 3745 persons died from lung cancer in 2012 of whom about 60% were aged 70 years or more.
Table II. Average annual number of deaths from lung cancer in Denmark, 1980–2012.
Adjusting for changes in population size and age, illustrates that mortality rates in women have increased steadily since 1980 for all age groups except for women aged 70–74 where the rates peaked around 2005 and then declined. In the age group 0–69 the rates rose until 1990 and have stabilized since. While the largest increase in mortality rates was observed in women aged 75–84, the mortality rates of those aged 80 years or more were lower than those aged 70–79. In men, a more complex pattern was seen (). The mortality rates decreased for men aged 70–79 years and increased among those aged 80 years or more.
Survival
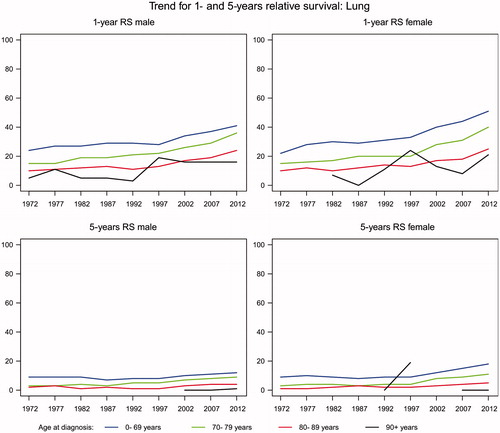
A trend for an improved one- and five-years relative survival for lung cancer was seen in both men and women and in all age groups (). Among men aged less than 70 years, the one-year relative survival increased from 24% to 41% and in women from 22% to 51% indicating that nearly 50% of the younger patients with lung cancer survived for one year. The one-year relative survival rates almost doubled in patients aged 70 years or more, but still only 25% of the patients aged 80–89 years survived their lung cancer for one year. The five-year relative survival is still very poor, around 10% for patients aged less than 80 years and 5% for those aged 80 years or more.
Discussion
This analysis demonstrates that the incidence of lung cancer is still increasing in Denmark for men among those older than 75 years and for women those older than 70 years. The incidence of lung cancer is closely linked to the pattern of tobacco smoking with the differences between age groups reflecting smoking behavior in birth cohorts. The high number of female smokers in Denmark in the 1970s explains the high incidence of lung cancer among elderly women in Denmark now [Citation8]. In 2012, the incidence of women with lung cancer are very close to the incidence of men, but the later peak in smoking exposure in women compared to men indicate that the plateau has not yet been reached in lung cancer incidence for women. The smoking epidemic has been analyzed by Lopez et al. [Citation9]. Based on 100 years observation in the countries with the longest history of tobacco smoking a four-stage model was described. Men began smoking, reaching exposures that exceeded 60% after about 50 years, and stayed at this level for about 20 years. Then the smoking exposure declined and stabilized at a level of 30%. Women began smoking about 20 years later and reached exposure levels of about 40%. Eventually, the exposure level stabilizes at about 20–30%. The mortality, which may be caused by smoking related lung cancer, is seen about 40 years later and at the same time when the number of smokers is decreasing. This is well correlated to smoking habits in Denmark. The proportion of male smokers in Denmark has also decreased. In the 1950s and 1960s, 70–80% of males smoked, but proportion has declined to below 30% in 2010. The proportion of female smokers peaked in the 1970s. The proportion of female smokers decreased from the late 1970s with a stronger decline from the 1990s. In 2010, it was under 30% [Citation10,Citation11].
Survival after lung cancer has improved over time though it is still very poor with 90% of the patients dying within the first five years after diagnosis. There are several plausible explanations for the improvement in survival of lung cancer patients. Delays in cancer diagnosis and treatment may be an important factor for prognosis [Citation12,Citation13]. A National Cancer Plan focusing on cancer prevention, early detection, and improved treatment was introduced in 2000 and updated in 2005 and 2011. Also a National Integrated Cancer Pathways in Denmark with a structured, fast and uniform detection and treatment for all patients with a suspicion of cancer was initiated in 2009 along with more aggressive therapy. Danish Lung cancer group was founded in 1997 to improve survival of lung cancer. Other explanations are the improvement in treatment in this period with the introduction of adjuvant chemotherapy in 2000 [Citation14] and platinum-based chemotherapy for stage III and IV lung cancer and the improvement in radiotherapy [Citation15]. Other improvements include the targeted therapy based on molecular analyzes for mutations and determination of specific targets as well as improvement in surgery [Citation16]. The prognosis for lung cancer patients depends on age, cancer stage, sex, and level of comorbidity [Citation2]. It is a limitation to this study that no information on comorbidity or stage was available. Comorbidity has been evaluated in a population-based cohort study in Denmark among lung cancer patients [Citation2]. Comorbidity was a negative prognostic factor for survival, and the improvement in survival was seen mainly among patients with no comorbidity. This may in part explain the inferior survival among the old patients, as they are expected to have more comorbidity. A high level of comorbidity will have negative impact on the treatment offered. Another limitation of this study is the lack of cancer stage at diagnosis. Some patients are diagnosed in an early stage, hence localized disease. Definitive treatment is possible and survival may improve compared to patients in advanced stage offered palliative treatment. However, more patients may be diagnosed with lung cancer because of the improvement in lung cancer detection in Denmark. This may result in more patients diagnosed with lung cancer too fragile to receive treatment. This will have a negative impact on survival. During the last decade, an improvement in imaging of lung cancer has led to improved staging as computed tomography (CT) scans and positron emission tomography (PET)-CT scans are more available. This will lead to a more correct staging but perhaps in a higher stage than with the imaging available in previous decades, where other imaging were available (stage migration). Another explanation can be that patients are more aware of the symptoms of cancer and will seek medical assistance earlier. This can lead to earlier staging of their lung cancer. It can be hypothesized that the improvement in one-year survival overall reflects the improvement in therapy for lung cancer.
In the last decade, the incidence and mortality from lung cancer has decreased in individuals aged 50 and younger, but it has increased in patients older than 70 years of age [Citation17]. At the same time the demographics in the Western world is shifting toward an older population. This combination has resulted in a continuously increasing number of elderly patients with cancer. This increase in incidence of lung cancer in the elderly is probably also related to the longer lifetime exposure to tobacco smoke and other carcinogens. Despite the high incidence of lung cancer in older patients, the elderly patients are underrepresented in clinical trials evaluating anti-cancer agents. As a consequence, a clinical uncertainty about treatment of older cancer patients exists [Citation18]. This may result in suboptimal treatment or excessively toxicity and thus lead to poorer outcomes compared with the outcome in younger patients. This may be part of the explanation for the high mortality among the 75 + with lung cancer. Renal and liver function and bone marrow reserves decline with age, and this could have an impact of the tolerability of anti-cancer treatment [Citation19]. A small number of trials have examined how elderly patients tolerate chemotherapy. The different outcome has been explained as diversity in comorbidity [Citation16,Citation20]. Carefully selected patients older than 70 years tolerate doublet chemotherapy and seem to have an improvement in overall survival [Citation21]. Others have concluded than monotherapy with chemotherapy was appropriate for treating elderly patients [Citation22]. However, addition of chemotherapy to radiotherapy among patients of 70 years and older with local advanced NSCLC may not increase survival [Citation23]. A number of studies evaluating treatment for the elderly have shown that older patients are less likely to receive surgery or other therapies [Citation16]. Radiotherapy has been shown to be effective and well tolerated in patients 80 years or older [Citation24] and especially stereotactic radiotherapy seems suitable for the elderly fragile patients [Citation15] leading to better results than what can be obtained by conventional radiotherapy [Citation25].
In conclusion, elderly patients with lung cancer are a heterogeneous group [Citation18]. A number of elderly very fit patients have excellent organ function and are in good performance status. It is important to treat this group optimally and not to exclude this group of patients from treatment because of age. In to select the most appropriate patients for treatment it has been suggested to use, for example, comprehensive geriatric assessment to evaluate patients functional age [Citation18].
Acknowledgements
We thank Niels Christensen and Anne Mette T. Kejs, Danish Cancer Society, Department of Documentation & Quality, for making the tabulations of incidence, mortality and prevalence, calculation of relative survival and trend graphs.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Deleuran T, Thomsen RW, Norgaard M, Jacobsen JB, Rasmussen TR, Sogaard M. Comorbidity and survival of Danish lung cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol 2013;5:31–8.
- Deleuran T, Sogaard M, Froslev T, Rasmussen TR, Jensen HK, Friis S, et al. Completeness of TNM staging of small-cell and non-small-cell lung cancer in the Danish cancer registry, 2004-2009. Clin Epidemiol 2012;4:39–44.
- Boyle P, Maisonneuve P. Lung cancer and tobacco smoking. Lung. Cancer 1995;12:167–81.
- Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev 2005;31:380–402.
- Ewertz M, Christensen K, Engholm G, Kejs A, Lund L, Matzen LE, et al. Trends in cancer in the elderly population in Denmark, 1980-2012. Acta Oncol 2015; doi:10.3109/0284186X.2015.1114678.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004;23:51–64.
- Barendregt JJ, Looman CW, Bronnum-Hansen H. Comparison of cohort smoking intensities in Denmark and the Netherlands. Bull World Health Organ 2002;80:26–32.
- Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco control 1994;3:242–7.
- Clemmensen KK, Lynge E, Clemmensen IH. Nationwide tobacco surveys and sales data in Denmark from 1920 to 2010. Dan Med J 2012;59:A4448
- Osler M. Smoking habits in Denmark from 1953 to 1991: a comparative analysis of results from three nationwide health surveys among adult Danes in 1953-1954, 1986-1987 and 1990-1991. Int J Epidemiol 1992;21:862–71.
- Hansen RP, Olesen F, Sorensen HT, Sokolowski I, Sondergaard J. Socioeconomic patient characteristics predict delay in cancer diagnosis: a Danish cohort study. BMC Health Serv Res 2008;8:49
- Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of primary lung cancer. Acta Oncol 2002;41:147–52.
- Vansteenkiste J, De RD, Eberhardt WE, Lim E, Senan S, Felip E, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi89–98.
- Haasbeek CJ, Palma D, Visser O, Lagerwaard FJ, Slotman B, Senan S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012;23:2743–7.
- Gajra A, Jatoi A. Non–small-cell lung cancer in elderly patients: a discussion of treatment options.. J Clin Oncol 2014;32:2562–9.
- Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer 2003;97:3133–275.
- Pallis AG, Gridelli C. Is age a negative prognostic factor for the treatment of advanced/metastatic non-small-cell lung cancer? Cancer Treat Rev 2010;36:436–41.
- Deppermann KM. Influence of age and comorbidities on the chemotherapeutic management of lung cancer. Lung Cancer 2001; 33:S115–20.
- Avery EJ, Kessinger A, Ganti AK. Therapeutic options for elderly patients with advanced non-small cell lung cancer. Cancer Treat Rev 2009;35:340–4.
- Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavole A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378: 1079–88.
- Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 2003;95:362–72.
- Hansen O, Schytte T, Nielsen M, Brink C. Age dependent prognosis in concurrent chemo-radiation of locally advanced NSCLC. Acta Oncol 2015;54:333–9.
- Bayman N, Alam N, Faivre-Finn C. Radiotherapy for lung cancer in the elderly. Lung Cancer 2010;68:129–36.
- Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552–8.