Abstract
Background Breast cancer is the most frequent malignancy among women worldwide and the second most common cause of cancer-related death in developed countries. The aim of the present analysis is to describe trends in incidence, mortality, prevalence, and relative survival in Denmark from 1980 to 2012 focusing on age, comparing persons aged 70 years or more with those aged less than 70 years.
Material and methods Cancer of the breast was defined as ICD-10 code C50. Data derived from the NORDCAN database with comparable data on cancer incidence, mortality, prevalence and relative survival in the Nordic countries, where the Danish data were delivered from the Danish Cancer Registry and the Danish Cause of Death Registry with follow-up for death or emigration until the end of 2013.
Results The proportion of patients diagnosed with breast cancer over the age of 70 years increased with time to 29% of women and 44% of men in 2012. Incidence rates increased with time and peaked around 2010 in all age groups except for those aged 90 years or more. Mortality rates were clearly separated by age with increasing mortality rates by increasing age group for both women and men. Relative survival increased over time in all age groups, but patients aged 70 years or more had a poorer relative survival than those aged less than 70 years. In 2012, 58 521 persons (all ages) were alive in Denmark after a diagnosis of breast cancer.
Conclusion Poorer survival of Danish breast cancer patients over the age of 70 years is likely to be due to inferior treatment and non-adherence to treatment guidelines. There is a need for clinical trials focusing on patients over the age of 70 years.
Breast cancer is the most frequent malignancy among women worldwide and the second most common cause of cancer-related death (after lung cancer) in developed countries [Citation1]. In Denmark, the incidence has been increasing steadily since the 1960s and the mortality has declined resulting in an improvement in survival [Citation2]. The improved prognosis is assumed to be the result of earlier diagnosis, particularly the use of mammography screening, and improved treatment, especially the use of more effective adjuvant therapy [Citation3].
Gender is the strongest risk factor for developing breast cancer with a male:female ratio of approximately 1:100 [Citation1]. The rarity of breast cancer in men has made it difficult to study etiologic factors. Recently, the Male Breast Cancer Pooling Project published results from 11 case-control and 10 cohort studies, including a total of 2405 male breast cancer cases and 52 013 controls without breast cancer, including 156 cases and 468 controls from the Nordic countries [Citation4]. The risk of men developing breast cancer increased with increasing recent BMI, a family history of breast cancer, Klinefelter syndrome, and medical conditions like diabetes mellitus, cryptorchidism, and a history of fractures [Citation4].
Age has a pronounced effect on the risk of developing breast cancer both in men and in women with increasing incidence rates with increasing age. The peculiar shape of the age-specific incidence curve with a “hook” or change in slope around age 50 in women was first described by Johannes Clemmesen, founder of the Danish cancer registry, in 1948 [Citation5]. It has been associated with a protective effect of menopause in women and lead to the hypothesis that risk factors may differ in pre- and postmenopausal women. In men, the age-specific incidence curve is a straight line of a constant increase with age [Citation6].
Apart from gender and age, the risk of developing breast cancer is increased in persons carrying mutations in the BRCA1 and BRCA2 genes. In women, reproductive factors, such as parity and late age at first birth, are associated with a higher risk in premenopausal than in postmenopausal women while risk estimates for increasing alcohol intake and BMI are higher for postmenopausal women [Citation7].
The aim of the present analysis is to describe trends in incidence, mortality, prevalence, and relative survival in Denmark from 1980 to 2012 focusing on age, comparing persons aged 70 years or more with those aged less than 70 years.
Material and methods
Cancer of the breast was defined as ICD-10 code C50. A more detailed description of the materials and methods appear elsewhere [Citation8]. In brief, data derives from the NORDCAN database with comparable data on cancer incidence, mortality, prevalence and relative survival in the Nordic countries, where the Danish data are delivered from the Danish Cancer Registry and the Danish Cause of Death Registry with follow-up for death or emigration until the end of 2013. This study focuses on the elderly population with age categorized as 0–69, 70–79, 80–89 and 90 + years.
For incidence and mortality, age group-specific numbers and rates per 100 000 person years are shown in tables and graphs with calendar periods for time of diagnosis 1978–1982, 1988–1992, 1998–2002, 2003–2007, 2010, 2011 and 2012. Prevalence is defined as the number of cancer patients (including cured patients as well) with that specific diagnosis still alive and is shown in tables by the end of 1980, 1990, 2000, 2005, 2010, 2011 and 2012.
Sex- and age-specific one- and five- year relative survival proportion ratios were calculated for each of the diagnostic groups for the age groups 0–69, 70–79, 80–89 and 90 + years and for the five-year periods of diagnosis 1968–1972, 1973–1977, …, 2003–2007 and 2008–2012.
Relative survival for a group of cancer patients is calculated as the observed survival (where all causes of death are considered events) divided by the expected survival of a group from the Danish population with the same age and year of birth composition. Actuarial method is used for observed survival and Ederer II method for expected survival [Citation9]. Relative survival can be interpreted as the survival if the cancer was the only cause of death. For the most recent period, 2008–2012, not all patients can be followed up for death in five years and we used hybrid methods where we supplement with survival experience from cancer patients diagnosed earlier years. Survival was not calculated for cancer groups with less than five patients [indicated by (-) in tables and blank in the graphs]. If all patients die in the follow-up period resulting in zero survival this is indicated as 0 (-) and if calculation for a cell results in a relative survival higher than 100% the result is shown in tables, but restricted to 100% in graphs.
Results
Incidence and mortality
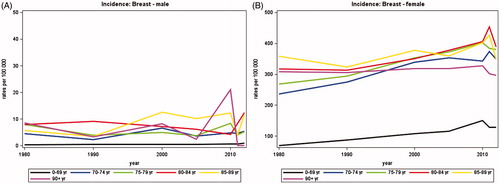
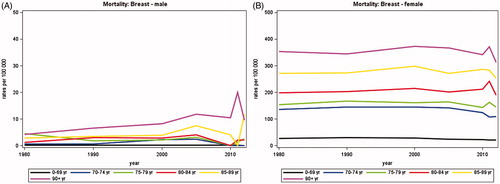
In women, the average annual number of newly diagnosed breast cancers increased from 2402 in 1980 to a maximum of 5018 around 2010 and then declined to 4474 in 2012 and in men, there was a steady increase from 21 in 1980 to 43 in 2012 (). The proportion of patients diagnosed over the age of 70 years increased with time to 29% of women and 44% of men in 2012 (). shows that the average annual number of deaths from breast cancer has decreased over time among women to 1123 in 2012.
Table I. Average annual number of new breast cancers in Denmark, 1980–2012.
Table II. Average annual number of deaths from breast cancer in Denmark, 1980–2012.
Adjusting for changes in population size and age, illustrates that no clear pattern in incidence rates were seen with age among men, probably due to the small number of patients. In women, the incidence rates increased with time and peaked around 2010 in all age groups except for those aged 90 years or more. The incidence increased with age, the rates among women aged 70 years or more being about three times higher than among those aged less than 70 years. The mortality rates () were clearly separated by age groups with increasing mortality rates by increasing age group for both women and men. There was no evident trend with time in the mortality rates.
Prevalence
Since 1980 the prevalence of breast cancer has been increasing substantially (). In 2012, 58 521 persons (all ages) were alive in Denmark after a diagnosis of breast cancer, 264 men and 58 257 women. The proportion of persons aged 70 years or more has also increased to 43% (25 361 of 58 521) in 2012.
Table III. Annual number of persons alive with breast cancer in Denmark by December 31, 1980–2012.
Survival
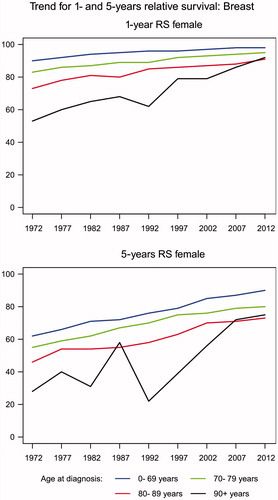
Age-specific relative survival is shown in for women only, as there were too few men to provide stable estimates. Both the one- and the five-year relative survival increased steadily over time for all age groups. Thus, for patients (0–69 years) diagnosed from 1968 to 1972, the five-year relative survival increased from 62% (95% CI 61–63%) to 90% (95% CI 89–90%) for those diagnosed from 2008 to 2012. Correspondingly, for patients (70–79 years) diagnosed from 1968 to 1972, the five-year relative survival increased from 55% (95% CI 52–58%) to 80% (95% CI 79–81%) for those diagnosed from 2008 to 2012.
With respect to age, both the one- and five-year relative survival decreased with increasing age group but the differences diminished with time. Thus, in the most recent period from 2008 to 2012, the relative five-year survival was similar for women aged 80–89 years (73%, 95% CI 70–75%) and women aged 90 years or more (75%, 95% CI 64–86%).
Discussion
Age has a pronounced influence on incidence and mortality from breast cancer in men and women with increasing rates with increasing age. The rarity of breast cancer in men precluded an analysis of survival but evidence from 1429 male breast cancer patients from the Nordic countries has shown that the relative survival was three-fold higher among men aged 80 years or more compared with those aged 40 years or less [Citation10]. It was concluded that important differences exist between male and female patients in the association between survival and age at diagnosis, possibly related to a later stage at diagnosis in men.
In women, the incidence of breast cancer increased from 1980 to 2010 and then declined while no clear trends were seen in the mortality rates, resulting in increasing survival over time. The peak in incidence just before 2010 among women aged less than 70 years can be explained from introduction of mammography screening. The first mammography screening program started in 1991 but covered only about 20% of Danish women aged 50–69 years. The national roll-out of mammography screening took place from 2008 to 2010 in Denmark [Citation11]. An equivalent peak in incidence was not observed for women aged of 70 years or more.
With the increasing incidence and the almost constant mortality, survival after breast cancer has increased over time for all age groups. However, when the mortality from other causes than breast cancer has been taken into account in the relative survival, women aged 70 years or more still have a poorer survival than those less than 70 years. These results are consistent with other studies [Citation12,Citation13]. As breast cancers among elderly women are associated with a more favorable tumor biology, more often being hormone receptor positive and human epidermal growth factor receptor-2 negative compared with younger women, the poorer survival is likely to be due to an inferior treatment. This has been documented in several studies, showing that women aged over 74 years were more likely to receive no treatment, surgery alone, or hormone therapy alone compared with younger patients, and that elderly patients were undertreated according to national guidelines [Citation14]. In Denmark, the national guidelines for adjuvant breast cancer treatment did not include patients over 75 years until 2002, and the guidelines for adjuvant radiotherapy still recommend an individual assessment of expected benefit versus harm among patients aged over 75 years.
There are several reasons why treatment guidelines do not include elderly patients. Guidelines are mostly based on evidence from randomized clinical trials and elderly patients with early breast cancer are underrepresented in such adjuvant trials [Citation15–17], e.g. the Early Breast Cancer Trialists’ Collaborative Group overview [Citation17] included only 651 patients aged 70 years or more of a total of 44 251 patients randomized to polychemotherapy with anthracyclines versus taxanes, i.e. 1.5%. The estimates of benefit from polychemotherapy among patients aged 70 years or more were similar or even greater than in younger patients, but less precise, i.e. not significant, probably because of the smaller number of patients, Second, randomized clinical trials often exclude patients with comorbidity and the incidence of comorbidities increases with age [Citation18]. In Denmark, comorbidity is present in approximately 26% of breast cancer patients [Citation19], and has a significant and independent impact on survival after early-stage breast cancer, with poorer survival among patients with one or more comorbid conditions [Citation19–21]. However, patients with mild to moderate comorbidity (Charlson Comorbidity Index of 1 and 2) receiving chemotherapy had similar breast cancer mortality as patients with no comorbidity [Citation20]. This indicates that the effects of chemotherapy in the elderly with mild/moderate comorbidity are similar to the benefits among patients with no comorbidity.
There is a substantial variation in breast cancer treatment by age, probably because of lack of knowledge about treatment effects in the elderly [Citation22]. Data collected from elderly women not treated in clinical trials show that clinician preferences influence the choice of adjuvant chemotherapy for elder patients [Citation23].
The average life expectancy for a Danish woman born today is 82 years [Citation24]. The decision about breast cancer therapy in elderly patients should take into account life expectancy, comorbidity, risk of breast cancer recurrence (i.e. disease stage and tumor characteristics), and functional status. Today, age alone should not be an appropriate criterion for treatment decisions. If at all possible, patients should be offered guideline therapy but it is necessary to monitor closely the toxicity as the elderly are frailer than younger patients [Citation25].
There is still a need of conducting trials evaluating the efficacy of less intense treatment among elderly breast cancer patients since incidence rates are high in this age group. Age over 70 years and co-existing comorbidities should be allowed in such trials to reflect the target population.
Conclusion
Danish breast cancer patients over the age of 70 years have a poorer prognosis with increased mortality rates and poorer relative survival compared to the younger patients. This is likely to be due to inferior or non-guideline therapy of the elderly patients. With the increasing longevity of breast cancer patients, more clinical trials are needed with particular focus on the elderly population.
Acknowledgment
We thank Niels Christensen and Anne Mette T. Kejs, Danish Cancer Society, Department of Documentation & Quality, for making the tabulations of incidence, mortality and prevalence, calculation of relative survival and trend graphs.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Globocan database. [cited 2014 August 11]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- NORDCAN database. [cited 2014 August 11]. Available from: http://www-dep.iarc.fr/NORDCAN/DK/StatsFact.asp?cancer=180&country=208
- Mouridsen HT, Bjerre KD, Christiansen P, Jensen MB, Møller S. Improvement of prognosis in breast cancer in Denmark 1977-2006, based on the nationwide reporting to the DBCG Registry. Acta Oncol 2008;47:525–36.
- Brinton LA, Cook MB, Mccormack V, Johnson KC, Olsson H, Casagrande JT, et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst 2014;106:1–11. doi: 10.1093/jnci/djt465.
- Clemmesen J. Carcinoma of the breast; results from statistical research. Br J Radiol 1948;21:583–90.
- Ewertz M, Holmberg L, Karjalainen S, Tretli S, Adami HO. Incidence of male breast cancer in Scandinavia, 1943-1982. Int J Cancer 1989;43:27–31.
- Brinton L, Smith L, Gierach GL, Pfeiffer RM, Nyante SJ, Sherman ME, et al. Breast cancer risk in older women: results from the NIH-AARP Diet and Health Study. Cancer Causes Control 2014;25:843–57.
- Ewertz M, Christensen K, Engholm G, Kejs AMT, Lund L, Matzen LE, et al. Trends in cancer in the elderly population in Denmark, 1980–2012. Acta Oncologica 2015;55: doi: 10.3109/0284186X.2015.1114678.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004;23:51–64.
- Adami HO, Hakulinen T, Ewertz M, Tretli S, Holmberg L, Karjalainen S. The survival pattern in male breast cancer. An analysis of 1429 patients from the Nordic countries. Cancer 1989;64:1177–82.
- Christiansen P, Vejborg I, Kroman N, Holten I, Garne JP, Vedsted P, et al. Position paper: Breast cancer screening, diagnosis, and treatment in Denmark. Acta Oncologica 2014;53:433–44.
- Ferguson NL, Bell J, Heidel R, Lee S, Vanmeter S, Duncan L, et al. Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast J 2013;19:22–30.
- Dialla PO, Quipourt V, Gentil J, Marilier S, Poillot ML, Roignot P, et al. In breast cancer, are treatments and survival the same whatever a patient's age? A population-based study over the period 1998-2009. Geriatr Gerontol Int 2015;15:617–26.
- Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud-Caspar I, Schäfer P, et al. Undertreatment Strongly Decreases Prognosis of Breast Cancer in Elderly Women. J Clin Oncol 2003;21:3580–7.
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–717.
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA JrJr, Albain KS. Underrepresentation of Patients 65 Years of Age or Older in Cancer-Treatment Trials. N Engl J Med 1999;341:2061–7.
- Early Breast Cancer Trialists' Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 2012;379:432–44.
- Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2001;285:885–92.
- Land LH, Dalton SO, Jensen MB, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990-2008. Breast Cancer Res Treat 2012;131:1013–20.
- Land LH, Dalton SO, Jensen MB, Ewertz M. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer 2012;107:1901–7.
- Land LH, Dalton SO, Jørgensen TL, Ewertz M. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol/Hematol 2012;81:196–205.
- Enger SM, Thwin SS, Buist DS, Field T, Frost F, Geiger AM, et al. Breast Cancer Treatment of Older Women in Integrated Health Care Settings. J Clin Oncol 2006;24:4377–83.
- Mandelblatt JS, Faul LA, Luta G, Makgoeng SB, Isaacs C, Taylor K, et al. Patient and Physician Decision Styles and Breast Cancer Chemotherapy Use in Older Women: Cancer and Leukemia Group B Protocol 369901. J Clin Oncol 2012;30:2609–14.
- Danmarks statistik (cited 2014 August 13). Available from: http://www.dst.dk/da/Statistik/emner/doedsfald-og-middellevetid/middel-levetid.aspx
- Tew WP, Muss HB, Kimmick GG, Von Gruenigen VE, Lichtman SM. Breast and ovarian cancer in the older woman. J Clin Oncol 2014;32:2553–61.