Abstract
Background and purpose Intensity-modulated radiotherapy (IMRT), also using volumetric modulated arc therapy (VMAT) and helical tomotherapy (HT) techniques, has been only recently introduced for treating anal cancer patients. We report efficacy and safety HT, and daily image-guided RT (IGRT) for anal cancer.
Materials and methods We retrospectively analyzed efficacy and toxicity of HT with or without chemotherapy for anal cancer patients. Local control (LC) and grade 3 or more toxicity rate (CTC-AE v.4.0) were the primary endpoints. Overall (OS), disease-free (DFS), and colostomy-free survival (CFS) are also reported.
Results Between October 2007 and May 2014, 78 patients were treated. Fifty patients presented a stage II or stage IIIA (UICC 2002), and 33 presented a N1–3 disease. Radiotherapy consisted of 36 Gy (1.8 Gy/fraction) delivered on the pelvis and on the anal canal, with a sequential boost up to 59.4 Gy (1.8 Gy/fraction) delivered to the anal and to nodal gross tumor volumes. Concomitant chemotherapy was delivered in 73 patients, mainly using mitomycin C and 5-fluorouracil (n = 30) or mitomycin C and capecitabine combination (n = 37). After a median follow-up period of 47 months (range 3–75), the five-year LC rate was 83.8% (95% CI 76.2–91.4%). Seven patients underwent a colostomy because of local recurrence (n = 5) or pretreatment dysfunction (n = 2). Overall incidence of grade 3 acute toxicity was 24%, mainly as erythema (n = 15/19) or diarrhea (n = 7/19). Two patients presented a late grade 3 gastrointestinal toxicity (anal incontinence). No grade 4 acute or late toxicity was recorded.
Conclusions HT with daily IGRT is efficacious and safe in the treatment of anal canal cancer patients, and is considered in our department standard of care in this clinical setting.
Anal canal cancer is a rare cancer. It represents 2% of all digestive cancers, and 6% of the ano-rectal cancers, but its incidence is increasing [Citation1]. External beam radiotherapy (EBRT), with or without concomitant chemotherapy (CT), is the standard of care in the treatment of squamous cell anal canal carcinoma. Randomized controlled trials (RCT) published in the 1990s definitely assessed the role of concomitant CT, in particular for locally advanced tumors [Citation2].
Optimal dose levels and schedules of EBRT are still under investigation [Citation1] but doses ranging between 45 and 59 Gy showed to be curative, with higher doses needed in poor responders patients [Citation1–4]. However, international recommendations support the delivery of a boost after the first course of EBRT with or without computed tomography (CT) delivered to the pelvic nodes and to the primary tumor [Citation1], which could be delivered using EBRT or brachytherapy (BRT) [Citation1,Citation2,Citation5,Citation6]. Published RCT showed an overall rate of non-hematological grade 3–4 acute toxicity of 54–74%, and an overall rate of non-hematological grade 3–4 late toxicity of 11–36% [Citation1,Citation2]. In all of the published RCT, EBRT was delivered using two-dimensional (2D)- or, at best, 3D-EBRT techniques.
Intensity-modulated radiation therapy (IMRT) is a complex technique, which preferentially targets structures while minimizing doses to adjacent normal critical structures. Several studies showed a reduction of toxicity rates in several cancer sites treated with IMRT compared to those treated with 3D-EBRT [Citation7–9]. Recently, more evolved forms of IMRT, such as volumetric modulated arc radiotherapy (VMAT) or helical tomotherapy (HT), have been introduced in the clinical practice. These irradiation techniques showed promising dosimetric improvements in some studies, when compared to “standard” 3D-EBRT or IMRT [Citation10–13]. Furthermore, the recent introduction of image-guided radiation therapy (IGRT) allowed online and offline daily verification of the setup of the patients. Using IGRT, the treatment volume can be reduced by reducing the size of the necessary margins taking into account for inaccuracies in target position and patient setup, with a consequent reduction of toxicity rates.
IMRT and IGRT have been recently adopted and prospectively evaluated also in the treatment of anal cancer patients, and results are promising in terms of local control (LC) and toxicity [Citation14–16]. However, the sample size of IMRT studies was often limited, with also short follow-up time (often <24 months).
In this study, we retrospectively analyzed efficacy and toxicity of IMRT with or without concomitant CT for anal cancer patients treated in our department. LC and grade 3 or more toxicity rates (CTC-AE v.4.0, [Citation17]) were the primary endpoints. Overall (OS), disease-free (DFS), and colostomy-free survival (CFS) rates are also reported.
Methods and materials
Population and treatment
We retrospectively reviewed data of patients with a histologically proven anal cancer consecutively treated with curative intent with EBRT with or without CT between October 2007 and May 2014. Follow-up of patients who had not been seen for more than 12 months were updated leading up to the date of this analysis. Tumors of the patients were retrospectively re-staged according to the 2002 International Union against Cancer Classification staging system (UICC 2002) [Citation18].
With these criteria, we identified 78 patients. Before the treatment, all of them received physical examination with digital rectal exploration (DRE) and ano-rectal echo-endoscopy. For the nodal and systemic staging, all of them underwent total body injected CT scan. Only recently, some of them presenting a clinical suspicion of locally advanced disease were staged with 18FDG positron emission tomography (PET)-CT (18 patients). Pelvic magnetic resonance imaging (MRI) was only rarely performed. Ten, 31, 19, 16, and two patients presented stage I, II, IIIA, IIIB, and IV disease, respectively. Median age was 61.9 years (range 38–87). summarizes the patients’ characteristics.
Table I. Population.
Patients underwent a visit with DRE and echo-endoscopy every three months in the first and the second year, every six months until the fifth year, and yearly thereafter. CT scan was performed every six months during the first five years, then yearly. Patients were followed in our department for at least two years after the end of the treatment. Then, those living far from our hospital continued their controls with their general practitioners, who were instructed about follow-up schedule adopted in our department and who readdressed us the patients in case of suspicion of relapse.
Target definition and treatment details
All patients received helical IMRT with HT to the anal canal and pelvic nodal areas. Anal canal, mesorectal, pelvic, and inguinal nodes received a total dose of 36 Gy (1.8 Gy/fr). The projection of the upper limit of pelvic fields was usually located at the L5-S1 level. All patients received prophylactic and/or curative EBRT on the inguinal nodes. Boost dose was delivered with either HT (n = 36, since 2011), or 3D-conformal EBRT (3D-CRT, n = 42). Target volumes for the sequential boost consisted of anal canal and the positive nodes. The median dose on this volume was 59.4 Gy (range 59.4–60 Gy), delivered with a median dose/fraction of 1.8 Gy (range 1.8–2 Gy/fr). Planning target volume (PTV) was obtained adding a 5-mm margin to the clinical target volume (CTV). Median overall treatment time (OTT) for EBRT with or without CT was 57 days (interquartile range 55–61). Following our internal protocols of treatment, a planned two-week break was adopted until 2011 (n = 15 patients). Then, an internal analysis of the toxicity rates has been performed, and showed a low rate of acute toxicity. Therefore, the planned gap was abolished for all the following patients. During treatment, all patients underwent daily IGRT using fan-beam computed tomography to check and correct their setup before the delivery.
The following constraints were used for treatment plan optimization: PTV: Volume receiving at least 95% of the prescribed dose = 95–98%; bowel (contoured as abdominal cavity)=Volume receiving at least 45 Gy (V45) < 195 cm3, V50 < 50 cm3; Femoral heads = Volume receiving at least 45 Gy <10%, Dose max (2 cm3) < 50 Gy; Bladder: Volume receiving at least 50 Gy <50%, dose max (2 cm3) < 55 Gy. A particular attention is given to the dose to the uninvolved skin and to genitalia, where the delivered dose was kept as low as possible.
Concomitant CT was delivered in 73 patients, most of whom (92%) received mitomycin C (10–15 mg/m2 given on the J1 and J29 of RT) combined either with 5-fluorouracil (5FU, 1000 mg/m2/day on the first five and last five days of RT, 41% of the patients) or capecitabine (825 mg/m2/day, two times a day, on the first five and last five days of RT, 51% of the patients).
Statistical analysis
Analysis included descriptive statistics of the whole population, including acute and late toxicity. We considered acute toxicity those recorded up to six months from the end of the treatment, while all others were considered as late toxicities. Primary endpoints were LC, and acute and late toxicity rates.
Acute and late toxicities were retrospectively scored using the Common Toxicity Criteria-Adverse Event score (CTC-AE v.4.0) [Citation17]. The definition of complete response was based on absence of any sign of tumor at the DRE and/or on the results of the anal echo-endoscopy. Proportions were compared by using the χ2-test for values of 5 or higher and with Fisher’s exact test for values of less than 5. Survival curves were estimated by using the Kaplan-Meier method [Citation19]. Time to any event was measured from the date of biopsy. Death certificates confirmed date of deaths. If clinical or pathologic evidence of active, recurrent disease was present, deaths were attributed to anal canal cancer. The events were death (all causes) for OS, death (all causes) or relapse for DFS, locoregional relapse for LC. Confidence intervals (CI) were calculated from standard errors. In univariate analyzes, differences between groups were assessed using the log-rank test [Citation20]. In multivariate analyzes, we screened for prognostic factors with a p value ≤0.20 in univariate analyzes using the Cox regression analysis to define the independent contribution of each prognostic factor. A p value of <0.05 was considered to be statistically significant. All data were examined using the JMP statistical software version 10 (SAS Institute Inc., Cary, NC, USA).
Results
Efficacy
Median follow-up period for the whole population was 47 months (range 3–75).
shows the OS, the DFS and the LC curves.
Figure 1. Overall survival (a), Disease-free survival (b) and local control (c) for the whole population.
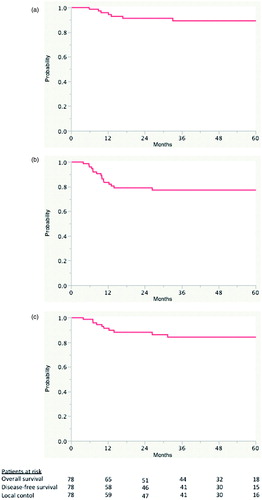
A total of 14 patients presented a recurrence (local only in five, locoregional in two, locoregional and distant in two, local and distant in three, regional only in one, and distant only in one patient). Median LC time was not reached, with a five-year LC rate of 84.2% (95% CI 74.9–93.1%). According to the technique used to deliver the boost (3D vs. HT), overall five-year LC was not statistically different: 89% versus 75%, respectively (p = 0.11). Median DFS time was not reached, with a five-year DFS rate of 77.2% (95% CI 68.6–85.8%). At the time of this analysis, seven patients died, with a five-year OS rate of 89.3% (95% CI 81.3–97.3%). Seven patients underwent a colostomy because of local recurrence (n = 5) or pretreatment anal dysfunction (n = 2). The CFS was 89.3% (95% CI 81.3–97.3%). Patients presenting also a nodal and/or a systemic relapse were treated with CT. The schedule and the type of CT were decided by the medical oncologist of the patient, following the internationally accepted standard treatment options for relapsing anal cancer patients.
summarizes the variables considered in the univariate analysis. and summarize results of univariate and multivariate analyzes (only the variables statistically influencing the considered endpoints are showed).
Table II. Variables considered in the univariate analysis.
Table III. Results of univariate analysis for local control and disease-free survival (only variables showing a p-value <0.05 in at least one of the considered outcomes are listed).
Table IV. Results of multivariate analysis for local control and disease-free survival (only variables independently influencing these endpoints are shown).
At multivariate analysis, the only factor confirming its independent influence on LC was the gender of the patient (see ). The stage of the disease (stage I–II vs. III–IV) showed a strong, but not statistically significant trend towards a better LC in patients with early tumors (p = 0.055). Concerning the DFS, the gender, disease stage (stage I–II vs. III–IV), tumor size (≤3 cm vs. >3 cm), and concomitant CT confirmed their independent influence on this endpoint.
Toxicity
Globally, compliance to the treatment was usually good, with all the patients having received the whole treatment, and with no major deviations to the planned schedules of the combined treatment. Overall, grade 3 acute toxicity was observed in 19 patients (24.3%), mainly as erythema (n = 15/19) or diarrhea (n = 7/19). At the time of analysis, 73 patients presented more than six months of follow-up, and were considered evaluable for late toxicity. Two of 73 patients present a late grade 3 gastrointestinal (GI) toxicity (anal incontinence). No late grade 3 skin toxicity was recorded. No grade 4 acute or late toxicity was recorded. No statistical differences in terms of severe toxicity were recorded comparing according to the boost technique (HT vs. 3D-EBRT). Age (≤75 years vs. >75 years) had no impact on the risk of developing severe acute or late toxicity.
Stage IV patients
Two patients presented a stage IV disease at diagnosis. One of these patients presented two liver metastases but, because of her relatively young age (61 years) and her good general condition, she was treated with curative intent with the volumes and dose levels described above. She received CRT with capecitabine (825 mg/m2, every 12 hours) and mitomycin C (10 mg/m2, in concomitance of the first day of the boost). No neoadjuvant CT was delivered before CRT. Three months after the end of combined treatment, she presented a complete local and systemic response. The other stage IV patient presented a L5 bone metastasis: she received also 54 Gy at this level during her treatment. Induction CT was not possible in this patient because of her age (78 years) and her comorbidities. These two patients are still alive and disease-free 43 and 66 months after the end of the treatment.
Discussion
In this study, we present one of the largest series reporting data on outcomes and safety of HT with concomitant CT in the treatment of anal cancer patients. Compared to historical series of patients treated with 2D- or 3D-techniques in the context of RCT, our results show an improvement of acute and late toxicity profile and, consequently, of patients’ compliance compared to conventional approaches.
One of the main priorities of introducing a new technique in the treatment of anal cancer should be a reduction of acute and/or late treatment-related toxicity, without affecting the efficacy of the treatment. Several dosimetric studies already showed that IMRT successfully allowed a reduction of the doses delivered to small bowel, femoral head, perineal skin, and genitalia compared to 3D-EBRT [Citation10–13]. These dosimetric improvements also translate in the clinical outcomes of anal cancer patients treated with IMRT [Citation14,Citation15]. RTOG 05-29 trial was designed to show whether IMRT with concomitant 5-FU and mitomycin C CT would decrease by at least 15% the combined rate of grade 2 or more acute GI and genitourinary (GU) toxicity, as compared to the 3D-EBRT and concomitant 5-FU and mitomycin C arm from RTOG 98-11 [Citation15,Citation21]. The primary endpoint was not met, but dose-painted IMRT (DP-IMRT) was associated with a significant reduction in acute grade 3 (or more) cutaneous (23% vs. 49%) and GI (21% vs. 36%) toxicity. A multi-institutional retrospective study by Salama et al. on the use of IMRT as part of combined treatment for anal canal cancer patients, reported a 15.1% rate of grade 3 acute GI toxicity [Citation22], which seems lower than the rate reported in the RTOG 98-11 trial [Citation21]. Kachnic et al. published in 2012 a retrospective series of 43 patients treated with DP-IMRT [Citation23]. These authors adapted the doses to the clinical situation of the patient, with lower doses (42 Gy to the pelvis and 50.4 Gy to the tumor, 28 fractions) delivered to early stage tumors and higher doses (45 Gy to the pelvis and 50.4–54 Gy, 30 fraction) in more advanced diseases. With this adapted approach, they reported rates of dermatologic, GI, GU of 10%, 7%, and 7%, respectively. Two-year LC, OS, CFS, and metastasis-free survival were 95%, 94%, 90%, and 92%, respectively. All these dosimetric and clinical data seem to support the idea that future prospective trials for anal canal cancer should include IMRT as standard EBRT modality. Our results compared favorably with the reported series. Overall, grade 3 acute toxicity was observed in 19 patients (24%), mainly as erythema (n = 16). Grade 3 diarrhea was recorded in 7/78 (8.9%), a rate which seems to be significantly lower than those reported in the published randomized trials, and comparing well with other series of IMRT in anal cancer patients [Citation15,Citation16]. No grade 4 toxicity was recorded in our patients. Noteworthy, it should be clearly underlined that a limit of our analysis is that the acute and late toxicity has been retrospectively scored, depending on the data available in the clinical charts of the patients. It could be an important bias, as it could potentially lead to an underestimation of the real rate of toxicity.
A possible argument against the introduction of very CRT techniques (such as IMRT) and/or against the use of narrower margins (as those used when daily IGRT is adopted) is that they could let to a higher risk of target missing and, therefore, increased local relapse. Our data do not support this assumption: after a median follow-up period of 47 months, our experience on 78 patients treated using HT combined with daily IGRT and concomitant CT showed a four-year LC of 83.8%, which is at least comparable to the LC rates reported in available prospective studies [Citation1,Citation2]. Nevertheless, we found a not statistically significant, but important difference in terms of five-year LC in favor of 3D-techniques (90% vs. 75% for 3D-EBRT vs. IMRT, p = 0.11). In order to understand this difference, we analyzed whether T classification (T1–2 vs. T3–4), nodal status (N0 vs. N+, or N0–1 vs. N2–3), and UICC 2002 stage influenced the choice of HT as preferred treatment modality. We found that N + patients (vs. N0 patients, 64% vs. 36%, p = 0.008) and N2–3 patients (vs. N0–1, 82% vs. 18%, p = 0.001) had been more frequently treated with HT, probably because it allowed better organ sparing in these more geometrically difficult situations. These unbalanced populations, with patients with more advanced diseases treated with HT, could probably explain our data.
OTT is of paramount importance in anal cancer. RTOG 92-08 study showed that patients treated with 3D-CRT and concomitant CT with a gap of two weeks (n = 20) presented OS and DFS rates inferior to those of the patients treated without gap (n = 46) (43% vs. 73% vs. 63% and 34%) [Citation24]. Also the rates of locoregional failure and post-chemoradiation colostomy were higher in patients treated with a gap (respectively, 29% vs. 15% and 25% vs. 15%). These rates were also higher compared to the five-year colostomy rate recorded in the RTOG 98-11 trial (10%), with no breaks required [Citation21]. However, these results should be taken with caution because the RTOG 92-08 study was not originally designed to compare the two treatment arms (with or without breaks). Noteworthy, the rates of acute toxicities for patients treated without free interval were higher. Deniaud-Alexandre et al. showed that patients treated with a break of >38 days presented a 10-year DFS rate inferior to those who were treated with a break <38 days (p = 0.0025) [Citation25]. The reason of these findings is probably related to the rapid tumor proliferation rate of squamous cell carcinoma cells of the anal canal. In our department, a planned two-week break was adopted in the standard treatment of anal cancer patients, as done in most part of the radiation oncology departments in 1990s and early 2000. In 2011, we performed an internal analysis of the toxicity rates, and we found a low rate of acute toxicity. Therefore, the planned gap was abolished for all the following patients. Anyway, OTT and gap duration did not show any impact on the outcomes of our patients, both at the univariate and at the multivariate analysis.
In our study, median OTT for the entire treatment was 57 days, higher compared to the RTOG 05-29 study (43 days, range 32–59), and to the RTOG 98-11 (49 days; range 4–100) [Citation15,Citation21]. Nevertheless, our four-year LC rate (83.8%) is at least comparable to those recorded in the major randomized trials, ranging between 64% and 68% [Citation1,Citation2]. Interestingly, in our analysis, an OTT of more than 57 (more or less than the median time of the whole population) or 60 days did not significantly decrease the LC. Nevertheless, we also found a quite interesting trend. A not statistically significant longer treatment time was seen in patients presenting G3 acute toxicity (p = 0.07). When the total treatment time was considered as a continuous variable in the model of the multivariate analysis, longer treatment times were significantly associated with better disease survival rates (p = 0.046), which seem in contrast with the results of the major randomized trials.
Two important aspects should be underlined to explain better our low not-cutaneous acute toxicity rates: the delivered doses and the daily use of IGRT. Doses adopted in our department were adopted taking into account the doses used in the RTOG 98-11 trial [Citation21] and could be considered relatively lower than those used in the other studies. We delivered 36 Gy in 1.8 Gy/fraction to the anal canal, mesorectal, pelvic, and inguinal nodes, and a sequential boost of 23.4 Gy in 1.8 Gy/fraction (total dose 59.4 Gy) to the anal canal and involved nodes. The dose to the larger volume was lower than the 45–50.4 Gy in 1.8 Gy/fraction used in the other published studies [Citation1,Citation2]. It could influence the risk of toxicity.
Moreover, all patients underwent daily IGRT using fan-beam CT to check and correct their setup. Hence, a margin of only 5 mm was added to the CTV to obtain the PTV. Even in the absence of direct comparison with previous reports, it could easily argue that our approach may reduce the treatment volumes, thus allowing the good toxicity profile reported in this series.
Conclusions
This long-term analysis showed that modern IGRT-HT is safe and effective in the treatment of patients with anal squamous cell carcinoma. Our data show a drastic reduction of non-hematological toxicity rate, without increasing the local or locoregional failures rates. Therefore, IGRT-IMRT is the standard of care in our institution for the treatment of anal cancer patients.
References
- http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#anal accessed online on 07/07/2015.
- Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol 2014;111:330–9.
- Ferrigno R, Nakamura RA, Dos Santos Novaes PE, Pellizzon AC, Maia MA, Fogarolli RC, et al. Radiochemotherapy in the conservative treatment of anal canal carcinoma: retrospective analysis of results and radiation dose effectiveness. Int J Radiat Oncol Biol Phys 2005;61:1136–42.
- Huang K, Haas-Kogan D, Weinberg V, Krieg R. Higher radiation dose with a shorter treatment duration improves outcome for locally advanced carcinoma of anal canal. World J Gastroenterol 2007;13:895–900.
- Gerbaulet A, Pötter R, Mazeron JJ, Meertens H, Van Limbergen E. The GEC ESTRO Handbook of Brachytherapy Bruxelles, 2002 pp. 505–14.
- Lestrade L, De Bari B, Pommier P, Montbarbon X, Lavergne E, Ardiet JM, et al. Role of brachytherapy in the treatment of cancers of the anal canal. Long-term follow-up and multivariate analysis of a large monocentric retrospective series. Strahlenther Onkol 2014;190:546–54.
- Gomez-Millan J, Fernández JR, Medina Carmona JA. Current status of IMRT in head and neck cancer. Rep Pract Oncol Radiother 2013;18:371–5.
- Bauman G, Rumble RB, Chen J, Loblaw A, Warde P, Members of the IMRT Indications Expert Panel. Intensity-modulated radiotherapy in the treatment of prostate cancer. Clin Oncol (R Coll Radiol) 2012;24:461–73.
- De Bari B, Fiorentino A, Arcangeli S, Franco P, D'angelillo RM, Alongi F. From radiobiology to technology: what is changing in radiotherapy for prostate cancer. Expert Rev Anticancer Ther 2014;14:553–64.
- Ugurluer G, Ballerini G, Moeckli R, Matzinger O, Bourhis J, Ozsahin M. Helical Tomotherapy for the treatment of anal canal cancer: a dosimetric comparison with 3D conformal radiotherapy. Tumori 2015;101:268–72.
- Cendales R, Vásquez J, Arbelaez J, Bobadilla I, Torres F, Gaitan A. IMRT, RapidArc® and conformal radiotherapy in the treatment of tumours of the anal canal. Ecancermedicalscience 2014;8:469. doi: 10.3332/ecancer.2014.469. eCollection 2014.
- Brooks CJ, Lee YK, Aitken K, Hansen VN, Tait DM, Hawkins MA. Organ-sparing Intensity-modulated radiotherapy for anal cancer using the ACTII schedule: a comparison of conventional and intensity-modulated radiotherapy plans. Clin Oncol (R Coll Radiol) 2013;25:155–61.
- Troussier I, Huguet F, Servagi-Vernat S, Benahim C, Khalifa J, Darmon I, et al. Management of locally advanced anal canal carcinoma with modulated arctherapy and concurrent chemotherapy. Cancer Radiother 2015;19:127–38.
- Han K, Cummings BJ, Lindsay P, Skliarenko J, Craig T, Le LW, et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys 2014;90:587–94.
- Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27–33.
- Vieillot S, Fenoglietto P, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, et al. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol 2012;7:45
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. 2009 May. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- Sobin LH, Gospodarowicz MK, Wittekind Ch. Eds. TNM Classification of Malignant Tumors, 7th ed. Wiley-Blackwell, Oxford, 2009.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn 1958;53:457–81.
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50: 163–70.
- Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, III, Thomas CR. Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914–21.
- Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 2007;25:4581–6.
- Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 2012;82:153–8.
- Konski A, Garcia M, Jr, John M, Krieg R, Pinover W, Myerson R, et al. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys 2008;72:114–18.
- Deniaud-Alexandre E, Touboul E, Tiret E, Sezeur A, Houry S, Gallot D, et al. Results of definitive irradiation in a series of 305 epidermoid carcinomas of the anal canal. Int J Radiat Oncol Biol Phys 2003;56:1259–73.
