Abstract
Background The aim of this study was to examine breast cancer (BC) incidence and mortality trends in Estonia during recent decades and to compare the pattern of these trends with other selected European countries and regions. We attempt to explain the findings in relation to changes in Estonian society and healthcare system.
Methods BC incidence (1985–2012) and mortality (1985–2013) data for Estonia were obtained from the Estonian Cancer Registry and Statistics Estonia. Data for selected European countries were obtained from the EUREG database. Joinpoint regression was used to analyze age-standardized rates in Estonia by age. For international comparison of incidence and mortality rates, we used scatterplot with 95% confidence ellipses and the mortality to incidence ratio.
Results The overall BC incidence continues to increase in Estonia, while mortality has been in decline since 2000. Both incidence and mortality trends varied considerably across age groups. Among women aged 60 years and older, BC incidence increased at a rate of nearly 3% per year. Significant decrease in mortality was seen only among women aged 50–59 years. Comparison of scatterplots between countries and regions revealed two clusters in Europe separated along the incidence axis. The correlation between incidence and mortality in Estonia changed its direction in the mid-1990s.
Conclusion In recent years, the dynamics of BC burden in Estonia has transitioned towards the high incidence–low mortality type model, which is characteristic to Western, Northern and Southern Europe. Although overall BC incidence is much lower in Estonia than in more affluent European countries, mortality from BC is still relatively high, particularly among elderly women.
Breast cancer (BC) is the most frequent cause of cancer death among women in the world as well as in Estonia [Citation1,Citation2]. Thanks to developed population-based cancer registration and vital statistics, incidence and mortality data are the most consistently collected information on cancer burden in Europe and elsewhere, made available by WHO and other databases [Citation1]. Cancer incidence primarily reflects the distribution of risk factors in population, and in case of BC, is heavily influenced by screening practices [Citation3]. Differences in incidence trends between countries or regions may provide evidence for prevention of cancer and act as an indicator of differences in primary prevention strategies, but variation in age distribution, case registration and level of health care should also be taken into account [Citation3,Citation4]. Mortality measures the impact of cancer as a synthesis of incidence and fatality [Citation1,Citation4].
In terms of avoidable mortality, BC is regarded as a malignancy amenable to health care, implying that even if the disease has developed, death can be prevented by preclinical detection and effective treatment of early disease. Declining mortality from BC in developed countries has been suggested to reflect increased effectiveness of health care [Citation5,Citation6], including improved treatment as well as earlier and more precise diagnosis [Citation7]. Effectiveness of BC screening in reducing mortality has sometimes been questioned [Citation8]. In Estonia, opportunistic mammography screening started in the second half of the 1990s and organized screening was introduced in 2004 [Citation9,Citation10]. Estonia reestablished its independence in 1991, followed by major social changes and the transition to open-market economy. Significant economic reforms initiated during the 1990s succeeded to reverse the decline in GDP, and life expectancy has started to increase steadily since 1994. The share of state expenditure on health care has fluctuated between 5% and 6% with some reduction during the economic crisis in the late 2000s [Citation11]. Recent studies in Estonia have shown noticeable decline in BC mortality and considerable increase in survival, but disparities with more affluent countries still persist [Citation12].
The objective of this study was to examine BC incidence and mortality trends in Estonia over the last decades and compare the pattern of these trends with other selected European countries and regions. We also attempted to explain the findings in relation to changes in society and healthcare system in Estonia.
Material and methods
The Estonian Cancer Registry (ECR), a population-based registry with nationwide coverage since 1968 [Citation13], provided data on all BC cases diagnosed in Estonia from 1985 to 2012. BC mortality data from 1985 to 1988 were obtained from the ECR; data from 1989 to 2013 and population data for the entire study period 1985–2013 were obtained from Statistics Estonia [Citation14]. Both incidence and mortality rates were age-standardized to world standard population [Citation15] and age-standardized rates were calculated for the age groups 30–39, 40–49, 50–59, 60–69 and ≥70 years.
To detect changes in time trends of BC incidence and mortality in Estonia, joinpoint analysis was performed for all ages and selected age groups with Joinpoint Regression Program (version 4.1.1.1) from the Surveillance Research Program of the US National Cancer Institute (http://surveillance.cancer.gov/joinpoint/). Using years as an independent variable, we identified the time segments separated at points of significant change in the log linear slope of the trend. The best fitting point in time that separated periods before and after the change is the so-called “joinpoint”. The optimal number of joinpoints was selected with Bayes information criteria. We also calculated the annual percentage of change (APC) for each separate linear segment, where negative APC indicates a decreasing and positive APC an increasing trend.
Age-standardized BC incidence and mortality data were obtained from the EUREG database (http://eu-cancer.iarc.fr/EUREG/Default.aspx) for selected European countries and regions with equal duration of available incidence and mortality data. We pooled EUREG data into groups to represent Western, Southern, Northern and Eastern Europe (Supplementary Table I, available online at http://www.informahealthcare.com). Data for Russia were obtained from the pertinent annual report [Citation16]. For comparative analysis, Estonian data were divided into two sub-periods: 1985–1995 and 1996–2012. The age-standardized mortality and incidence rates per year for countries and regions were shown jointly on scatterplot as point coordinates. Corresponding 95% confidence ellipses were drawn around the resulting data point clouds. The association between mortality and incidence was tested with Spearman’s rank correlation in order to complement the visual inspection of scatter plots. The conjoint changes in incidence and mortality over time were depicted using the mortality to incidence ratio (MIR) subtracted from 1 and multiplied by 100 (1–MIR) [Citation17].
Table I. Age-standardized (world) breast cancer incidence (1985–2012) and mortality (1985–2013) rates per 100 000 by age group (years) in Estonia.
Confidence ellipses and Spearman’s rank correlation coefficients were calculated with Stata 12.1 (StataCorp LP, TX, USA).
The study protocol was approved by the Tallinn Medical Research Ethics Committee.
Results
Incidence
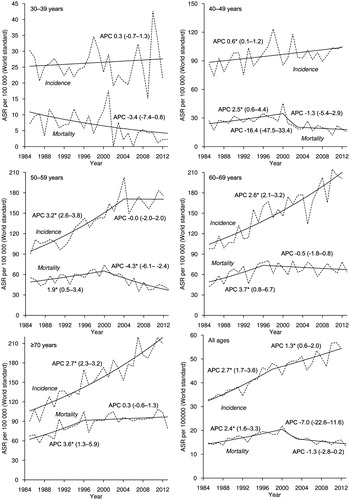
During 1985–2012, 15 284 incident cases of invasive BC were registered in Estonia (). The overall age-standardized incidence increased by 2.7% annually until 1998, after which the upward trend continued at a slower rate (). In the age group 30–39, no particular trends were seen. Among women aged 40–49, a slight but significant increase in incidence was observed. In the age group 50–59, the incidence rate increased until reaching the peak in 2004 and leveled off thereafter. Age groups 60–69 and ≥70 displayed a steep upward incidence trend for the whole study period.
Mortality
A total of 6647 BC deaths were recorded during 1985–2013 (). BC mortality for all ages increased steadily until 2000, then decreased sharply until 2003 and leveled off thereafter (). In the age group 30–39, mortality has decreased continuously. Mortality trend for the age group 40–49 was similar to that for all ages with two joinpoints in 2000 and 2003. In the age group 50–59, the statistically significant increase was replaced in 2000 by a significant decline. Mortality among older age groups 60–69 and ≥70 increased steeply until 1996 and reached a plateau thereafter.
International comparison
The scatter plot of BC mortality and incidence () revealed two distinctive clusters, separated along the age-standardized incidence axis. These may be roughly identified as Eastern (consisting of Eastern Europe, Estonia and Russia) and Western clusters (Southern, Western and Northern European countries). In Northern and Western Europe, the general correlation was from high mortality and low incidence to low mortality and high incidence, suggested by the oblong shape of the confidence ellipses sloping to the right, and strong negative correlation between incidence and mortality (). Similar shape appeared also for Eastern Europe and Estonia after 1995, albeit on lower incidence level. Southern Europe displayed a weak negative correlation between incidence and mortality. Russia showed increase in incidence but no change in mortality. For Estonia, the shape of the confidence ellipse for 1985–1995 showed inverse direction, characterized by strong positive correlation.
Figure 2. Breast cancer incidence and mortality in Estonia and other selected European countries and regions: a) Scatterplot of age-standardized incidence rates (ASIR) and mortality rates (ASMR) with 95% confidence ellipses and Spearman’s correlation coefficients; b) time trends of the mortality to incidence ratio (1–MIR). *p-value <0.05.
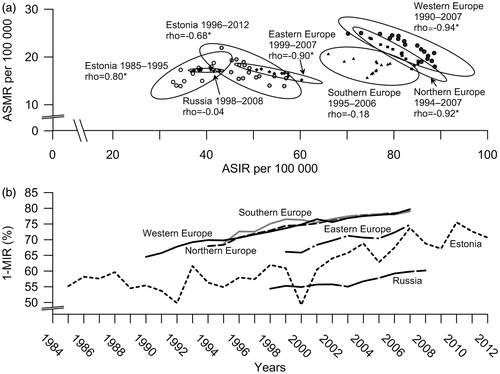
Changes in proxy survival measure 1–MIR () in Europe confirmed the general shift over time from low incidence–high mortality to high incidence–low mortality. For Estonia this pattern, although on lower level of incidence, was apparent since 1995.
Discussion
The relationship between BC incidence and mortality in Estonia during the study period clearly changed its direction over time and in the recent period started to resemble the pattern observed in more developed countries. This change most likely reflects the major social changes taking place in Estonia after regaining independence in 1991, followed by the adoption of Western lifestyle and transition to insurance-based health care. By the second half of the 1990s, major health care reforms had been implemented and modern mammography equipment had become increasingly available. At the same time, consensus guidelines were being gradually adopted in oncology.
This is the most comprehensive analysis of concurrent BC incidence and mortality trends in Estonia covering the period of transition. The main strengths of this study were the use of population-based cancer registry data collected uniformly over the study period and the comparison of BC trends in Estonia with other countries in Europe. This comparison was somewhat restricted by the limited availability of incidence and mortality data from the EUREG database; therefore the time series for selected regions and countries covered periods with different length. Still, there is substantial overlap in time and overall trends observed in our study do not contradict trends in incidence and mortality described in other studies [Citation2]. Due to the small population of Estonia the rates of BC occurrence are prone to larger fluctuation, especially in younger age groups. Joinpoint regression smoothed the random variation of rates in addition to finding the significant points of change in BC trends.
Increase in BC incidence has been observed since the beginning of cancer registration in Estonia in 1968 (http://eu-cancer.iarc.fr/EUREG/Default.aspx). It can be attributed to changes in reproductive and other important factors, such as excess weight, physical activity, alcohol consumption and breast feeding [Citation2,Citation7]. During the 1990s, the fertility rate among women of reproductive age started to decline rapidly and the average age at first birth increased [Citation14]. Health behavior studies have indicated that excess weight, obesity and low physical activity have increased among women aged over 45 years, while a rise in frequent alcohol consumption has been observed among women of younger age [Citation18]. The use of hormonal replacement therapy in Estonia was very low until the end of the 1990s, increased in the beginning of the 2000s, but declined again afterwards [Citation19,Citation20]. Wider use of mammography, including screening, is another plausible cause for the observed change in BC incidence. Due to early detection of small, less aggressive and slower growing tumors screening, as a rule, increases the incidence of BC and should theoretically lower mortality after 5–8 years [Citation7]. In addition, mass screening activities are usually accompanied by raised public awareness [Citation4]. Organized nationwide mammography screening in Estonia started in 2004, but it was preceded by pilot projects in two larger cities in 1996 and 1998, and a preparatory phase of screening from 2002 [Citation10]. The target age group was 50–59 years until 2007 and 50–62 thereafter [Citation12]. The main problems with organized screening in Estonia are its availability only to women with current health insurance (according to studies of health behavior among adult population in Estonia the proportion of women having health insurance in the age group 50–59 increased from 82% in 2002 to 95% in 2014) and low participation rate (50% in 2005–2006) [Citation9,Citation12,Citation18].
Our analysis showed that BC incidence increased in all age groups except for the youngest, although at a different rate. While the increase was very modest in the age group 40–49, it was quite dramatic for women age 60 years and over. In the age group 50–59, which is targeted by screening, the steep rise leveled off after 2004, which does not represent a typical screening curve, probably associated with low coverage. Mortality in this age group started to decline significantly already since 2000 – about four years before the start of organized screening. Considering that it takes about five years for incidence and mortality to become stable after screening [Citation21], it is difficult to attribute the observed decrease in mortality to the impact of organized screening, although opportunistic screening can obscure the effect of organized screening [Citation22]. It was shown previously that the proportion of localized cancers in age group 50–59 increased from 33% in 1995–1999 to 46% in 2000–2004. At the same time, the five-year relative survival of locally/regionally spread BC increased from 59% to 69%, suggesting major advances in treatment [Citation12]. The incidence/mortality pattern in this age group most likely reflects the combined effect of increased awareness, earlier diagnosis and improved treatment already during the prescreening period. The same factors probably also contributed to the mortality decline among younger women. However, mortality has not declined for women 60 years or older, indicating that they have benefitted less from aforementioned developments, compared to their younger counterparts. The survival disparity by age was particularly evident for non-localized tumors in Estonia [Citation12].
A study based on GLOBOCAN 2008 data demonstrated that on regional level, a weak positive association exists between BC mortality and incidence in Europe. An East-West differentiation was evident between countries along the diagonal that denotes low incidence–high mortality and high incidence–low mortality [Citation23]. In our analysis, the main differentiation appeared along the incidence axis, indicating the evolution of disease occurrence primarily from low incidence to high incidence in countries and regions in various stages of cancer control activities. Although the total health expenditure in Estonia rose rapidly during the 2000s, it is still well below that of countries in Western Europe [Citation24]. In this regard, the trends in BC incidence/mortality relationship observed in Estonia are not unique. Similar trends for a comparable period were observed in Eastern European countries, such as Czech Republic, Poland [Citation1,Citation2,Citation7] and Lithuania [Citation25].
In conclusion, BC continues to be an important public health issue in Estonia. Since the mid-1990s, the dynamics of BC burden has clearly transitioned towards the high incidence–low mortality type model, which is typical for Western, Northern and Southern Europe. Although the overall BC incidence is much lower in Estonia than in more affluent European countries, mortality from BC is still relatively high, particularly among elderly women. Measures to avoid premature deaths from BC should combine early detection and improved treatment.
Supplementary_table_baburin_et_al.docx
Download MS Word (25.2 KB)Acknowledgments
The study was supported by the Estonian Science Foundation (grant no ETF8881) and the Estonian Research Council (IUT5-1).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, et al. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol 2013;24:2657–71.
- Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer 2015;51:1164–87.
- Gatta G, Rossi S, Capocaccia R. Cancer burden estimates and forecasts: uses and cautions. Tumori 2013;99:439–43.
- Parkin DM, Fernández LMG. Use of statistics to assess the global burden of breast cancer. Breast J 2006;12:70–81.
- Nolte E, McKee M. Does health care save lives? Avoidable mortality revisited. London: The Nuffield Trust; 2004.
- Paap E, Holland R, Heeten GJ, Den Van Schoor G, Botterweck AAM, et al. A remarkable reduction of breast cancer deaths in screened versus unscreened women: a case-referent study. Cancer Causes Control 2010;21:1569–73.
- Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer 2008;44:1345–89.
- Autier P, Koechlin a, Smans M, Vatten L, Boniol M. Mammography screening and breast cancer mortality in Sweden. J Natl Cancer Inst 2012;104:1080–93.
- Ulp S, Kuusemäe K, Talk M, Raudsepp T. 10 aastat rinnavähi sõeluuringut Eestis: samm-sammult püstitatud eesmärkide poole. Eesti Arst 2010;89:493–501.
- Innos K, Mägi M, Tekkel M, Aareleid T. Place of residence predicts breast cancer stage at diagnosis in Estonia. Eur J Public Health 2011;21:376–80.
- Lai T, Habicht T, Kahur K, Reinap M, Kiivet R, van Ginneken E. Estonia: health system review. Health Syst Transit 2013;15:1–196.
- Baburin A, Aareleid T, Padrik P, Valvere V, Innos K. Time trends in population-based breast cancer survival in Estonia: analysis by age and stage. Acta Oncol 2014;53:226–34.
- Rahu M. Estonia. In: Parkin D, Muir C, Whelan S, Ferlay J, Raymond L, Young J, editors. Cancer incidence in five continents Vol. VII. Lyon: IARC; 1997, p. 466–9.
- Statistics Estonia/Statistikaamet. Statistical database/Statistika andmebaas http://pub.stat.ee/px-web.2001/dialog/statfile2.asp [Accessed 18th May 2015].
- Waterhouse J, Muir C, Correa P, Powell J, editors. Cancer incidence in five continents. Lyon: IARC; 1976.
- Chissov VI, Starinskiy VV, Petrova GV, editors. Malignant neoplasms in Russia in 2008 (incidence and mortality). [In Russian.] Moscow: P.A. Herzen Moscow Oncology Research Institute; 2010.
- Asadzadeh Vostakolaei F, Karim-Kos HE, Janssen-Heijnen MLG, Visser O, Verbeek ALM, Kiemeney LALM. The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur J Public Health 2011;21:573–7.
- Tekkel M, Veideman T. Health behavior among Estonian adult population, 2012. Tallinn: National Institute for Health Development; 2013.
- Kirss F, Lang K, Tuimala R. Feminine life-course of Estonian women born in 1937-47: a questionnaire survey. Acta Obstet Gynecol Scand 2006;85:224–8.
- State Agency of Medicines. Eesti ravimistatistika 2006–2010/Estonian Statistics on Medicines 2006–2010. Tartu: 2011.
- Anttila A, Sarkeala T, Hakulinen T, Heinävaara S. Impacts of the Finnish service screening programme on breast cancer rates. BMC Public Health 2008;8:38
- Moss SM, Nyström L, Jonsson H, Paci E, Lynge E, Njor S, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of trend studies. J Med Screen 2012;19: 26–32.
- Ades F, Senterre C, de Azambuja E, Sullivan R, Popescu R, Parent F, et al. Discrepancies in cancer incidence and mortality and its relationship to health expenditure in the 27 European Union member states. Ann Oncol 2013;24:2897–902.
- World BankHealth expenditure per capita http://data.worldbank.org/indicator/SH.XPD.PCAP/countries/1W?display=default [accessed 2nd June 2015].
- Krilaviciute A, Smailyte G, Brenner H, Gondos A. Cancer survival in Lithuania after the restoration of independence: rapid improvements, but persisting major gaps. Acta Oncol 2014;53: 1238–44.