Abstract
Background Registration of haematological malignancies presents specific challenges, and a wide range of data is required to ensure case ascertainment and proper classification of these diseases. We studied the data quality of myeloproliferative and myelodysplastic neoplasms in the Finnish Cancer Registry (FCR), comparing information with hospital discharges. Material and methods Hospital discharges (HILMO) in 2007–2013 including diagnostic codes of myeloproliferative and myelodysplastic neoplasms were extracted. Patients were individually linked to the FCR database for all haematological malignancies registered in 1953–2013. Coverage and accuracy of the FCR and agreement between registers was estimated. Results In total 5289 individuals were retrieved from two registers. Of these, 1406 were common, 1080 only found in the FCR and 2803 only in the HILMO. Coverage of myeloproliferative and myelodysplastic neoplasms in the FCR was 47.0% (95% CI 45.7–48.4%). Almost one quarter of the registrations in the FCR was based on a death certificate only. The accuracy of diagnosis was 51.4% (95% CI 49.4–53.3%), but it varied substantially by disease category. Kappa statistic for agreement between registers was excellent (0.83, 95% CI 0.80–0.85) for common cases. 7.6% of cases in the HILMO was registered as leukaemias in the FCR. Conclusions More than half of the patients found in the HILMO were entirely missing from the FCR. However, some of the diagnoses in HILMO may be preliminary and this represents the maximal number of missing cases. Cancer registers benefit from supplementary data sources, such as hospital discharges, to increase coverage and accuracy of register data on haematological malignancies.
Unlike many other cancers, haematological malignancies are diagnosed using a combination of histology, cytology, immunophenotyping, cytogenetics, imaging, and clinical data. This range of data is difficult to access systematically, but it is required to ensure complete case ascertainment and accurate morphological classification [Citation1]. Haematological malignancies present challenges for cancer registration also because they often progress or transform into other types of haematological malignancies, and for some diseases follow-up of several years or decades may precede a definite diagnosis. The aim of this study was to assess coverage and accuracy of myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS) in the Finnish Cancer Registry (FCR), comparing information in the FCR with administrative hospital discharge diagnoses [Care Register for Health and Welfare (HILMO)].
Material & methods
Finnish Cancer Registry (FCR)
The FCR records data on persons residing in Finland who have developed cancer since 1953 (www.cancer.fi/syoparekisteri/en). The process of coding cancers has followed the third edition of the International Classification of Diseases for Oncology (ICD-O-3) and it’s updates since 2007 [Citation2]. Cancer notifications are received from hospitals, physicians and pathology and haematology laboratories. Healthcare authorities, institutions and personnel are obliged to report cancer cases to the FCR without any consent of the patient by the Act on National Personal Records Kept under the Health Care System (556/1989) (www.finlex.fi). In addition, information on all death certificates with cancer mentioned is retrieved from the Statistics Finland once per year. The FCR aims to collect several notifications for each cancer case. If a clinical notification is missing, a reminder will be sent to the treating hospital.
Due to different information sources and active manual work in reviewing and compiling the data, the cancer register is fairly complete with regards to invasive solid tumours [Citation3,Citation4]. However, marked under-registration of haematological malignancies was evident already in 1980s varying from 10% to 45% [Citation3]. After that, an updated classification with new disease entities was published, and some diagnostic codes of haematological malignancies previously classified as neoplasms of uncertain behaviour changed into malignant codes [Citation5]. Also, new coding rules have been introduced [Citation6].
Finnish hospital discharges, HILMO
Care Register for Health and Welfare (formerly the Hospital Discharge Register, HILMO in this paper) includes data from hospitalisations (since 1969), day-surgical procedures (since 1994) and all outpatient visits in the public sector (since 1998). In the HILMO, every contact to the healthcare service has been recorded with International Classification of Diseases, 10th version (ICD-10) diagnosis from 1996 onward. A systematic review of validation studies using external data sources showed that the quality of HILMO data varies from satisfactory to very good [Citation7].
Linkage procedure
All citizens residing permanently in Finland have a unique personal identity code which allows individual level linkage between data from various sources.
We defined MDSs and MPNs based on the World Health Organization (WHO) 2008 classification [Citation5], the Haemacare manual [Citation6] and the European Network of Cancer Registries recommendation [Citation1]. The major difference between the WHO classification and the structure of the ICD-10 main categories is that the WHO categorisation of MDSs andMPNs also includes some leukaemias (see Supplementary Table I, available online at http://www.informahealthcare.com).
From the HILMO, all hospitalisations and outpatient visits from 2007 to 2013 with ICD-10 diagnostic codes of interest (Supplementary Table I) were extracted. The time period was chosen because of an updated WHO 2008 classification, and because of the majority of diseases studied here have been registered in the FCR only since 2007 (Supplementary Table I). The data extraction amounted initially to 21 946 independent admissions involving 4827 individuals. From the HILMO, we chose the earliest date of admission that included any of the eligible ICD-10 codes. In all, 14 people could not be linked to the FCR database due to an erroneous personal identity code. For others, all haematological malignancies (ICD-O-3 morphologies from M9590 to M9992) registered between 1953 and 2013 were obtained. From the FCR, only the first haematological malignancy recorded was counted as an incident case here. Whenever a patient had the same diagnosis date for multiple MPNs and/or MDSs, we included the one with a higher morphology code.
Statistical analyses
Coverage of cases registered in the FCR was estimated using the pooled number of cases from either of the two registers as the denominator. Accuracy of diagnosis was estimated using the proportion of register-detected cases that were confirmed to be true positives (positive predictive value) according to the diagnoses in the HILMO. Agreement between two registers was estimated by pair-wise Cohen’s kappa statistic and the 95% confidence interval (CI) was estimated using standard normal approximation of CI. We also evaluated incidence trends of MDSs and MPNs registered in the HILMO and in the FCR independently over the period 1996–2013. Analyses were performed using Stata (version 12.1, StataCorp, College Station, TX, USA) and R (version 3.2.0).
Results
Between 2007 and 2013, overall 5289 individuals with diagnosis of MDSs or MDNs were retrieved from two national independent healthcare registers. Of these, 1406 (26.6%) were common in both registers, 1080 (20.4%) only found in the FCR and 2803 (53.0%) only in the HILMO.
All 4813 individuals who had a diagnosis of MPN, myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MD/MPN) in the HILMO between 2007 and 2013 were matched to the entire FCR database (). Of these, 604 cases were registered in the FCR already before year 2007 and were excluded from the study material. In all, 2253 cases identified from the HILMO were entirely missing from the FCR. In total 550 persons were registered with some other haematological malignancy with almost one third registered before year 2007. Altogether 7.6% of MPN, MDS or MD/MPN cases reported by the HILMO between 2007 and 2013 were registered as leukaemia cases in the FCR. Of leukaemias, two thirds were acute myeloid leukaemias.
Table I. MPN, MDS or MD/MPN records reported by the HILMO in 2007–2013 matched by cross-referencing against the entire Finnish Cancer Registry database (n = 4813).
For accuracy and coverage, we compared data from the FCR and the HILMO (). Coverage in the FCR for all MDSs and MPNs was 47.0% (95% CI 45.7–48.4%), whereas the coverage in the HILMO was 79.6% (78.5–80.7%) when using the pooled number of cases from either of the two registers as a reference population. From the 3066 cases of MPN (registered either in the FCR or in the HILMO), the FCR recognised 1558. This translates to a coverage of 50.8% (95% CI 49.0–52.6%). For the MDS and for the MD/MPN the corresponding coverage estimates were 37.5% (35.4–39.5%) and 67.1% (59.1–74.3%), respectively. In contrast, the HILMO recognised 69.9% (68.3–71.5%) of MPN cases, 91.4% (90.2–92.6%) of MDS cases but only 37.3% (29.8–45.4%) of MD/MPN cases.
Table II. Number and type distribution of reported myeloproliferative and myelodysplastic neoplasms in two independent healthcare registers between 2007–2013 (n = 5289).
The proportion of FCR cases that were similarly diagnosed (true positives) in the HILMO was 51.4% (95% CI 49.4–53.3%). In addition 5.2% (4.4–6.1%) of diagnoses were matched by MDS and MPN of different type. The accuracy of diagnosis varied among the three disease categories with the positive predictive value being 40.8% (95% CI 38.4–43.4%) for the MPN, 77.1% (74.1–80.0%) for the MDS and 6.6% (2.7–13.1%) for the MD/MPN (). The overall agreement between the FCR and the HILMO over the three categories of MPN, MDS and MD/MPN was 0.83 (95% CI 0.80–0.85) in cases found from both registers.
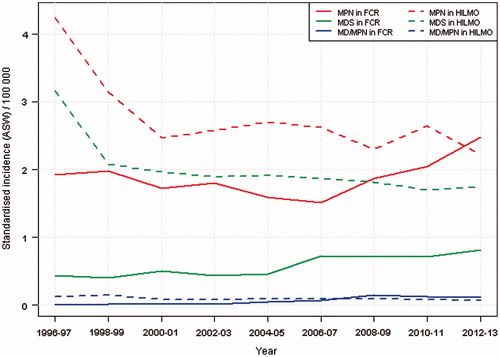
Two-year averages of the age-standardised (world) incidence rate of MPN has steeply increased since 2006 in the FCR, and incidence reached the level of the HILMO by the end of the study period (). Also the incidence of MDS in the FCR was increasing but the rate was substantially lower than that in the HILMO throughout the years.
Figure 1. Two-year averages of age-standardised incidence of myeloproliferative and myelodysplastic neoplasms in two independent healthcare registers in 1996–2013. FCR, Finnish Cancer Registry; HILMO, Care Register for Health and Welfare; MD/MPN, myelodysplastic/myeloproliferative neoplasms; MDS, myelodysplastic syndromes; MPN, myeloproliferative neoplasms.
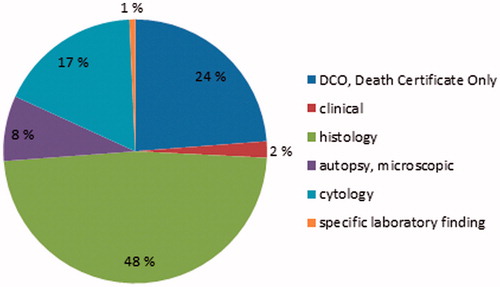
The most valid basis of diagnosis in cancer registration is the original (pre-treatment) histological diagnosis of the primary tumour. A total of 1195 of 2486 MPN, MDS and MD/MPN cases (48%) were registered in the FCR based on histological examination of tissue (). The proportion of all microscopically verified myeloproliferative and myelodysplastic neoplasms (histology, cytology or autopsy) was 73.5%. In almost one quarter of cases the basis of diagnosis was a death certificate only.
Discussion
We studied the data quality of myeloproliferative and myelodysplastic neoplasms in the FCR comparing information with hospital discharge data. The overall degree of coverage in the FCR was approximately 47%, varying from 37% for the MDSs to 67% for the MD/MPNs. The accuracy of diagnosis overall was 51% but it varied from 7% to 77% among the three disease categories.
Nordic countries have a long tradition of thorough record keeping, and all these population-based cancer registries are of high coverage and quality [Citation8]. Still already in the 1980s, registration of non-solid tumours was found to be significantly lower than that of solid tumours in all Nordic countries, which rely on passive reporting of cancer cases [Citation3,Citation9]. Active trace-back system based on the Norwegian Patient Register has significantly improved the quality of multiple myeloma registration in the Norwegian Cancer Registry [Citation10]. The completeness of multiple myeloma has increased from 92.7% in the 1990s to 95.5% in 2005, but under-reporting of haematological malignancies still remains. More recent studies also from Iceland and Denmark have demonstrated under-reporting of haematological malignancies in population-based cancer registries [Citation11,Citation12].
Under-reporting leads to an underestimation of both haematological malignancies and also of all cancers combined in summary statistics. Moreover, altogether 8% of individuals reported by the HILMO were registered mainly as acute myeloid leukaemias in the FCR. This indicates that transformed haematological malignancies contribute to the incidence of acute leukaemia in the Finnish statistics. This causes bias also in the survival estimates of acute leukaemia patients as transformed cases often have a very poor prognosis with a median survival of only few months [Citation13–15].
The coverage of MDSs and MPNs in the FCR was extremely poor, and unstable incidence rates over time support the observed under-reporting. The registration problems are less evident for MPNs than for MDS which is likely explained by differences in record keeping history (Supplementary Table I). Also, as MDS have been registered only since 2007, patients diagnosed earlier are missing from the FCR. It is worth noticing that the number of registered MDSs and MPNs in the FCR is now increasing (). This may be result of improved diagnostics during more recent years, changes in criteria for coding and registration and also better notification by hospitals and laboratories.
So far, we have not checked the cases missing from the FCR from hospital records. Thus, the reported deficiency represents the maximal amount of missing diagnoses. Plausible explanation for under-reporting is that morphology codes used in laboratories do not follow the current new WHO classification, or data extraction rules used to report haematological malignancies may be inadequate. Haematologic diagnoses sometimes lack the pathology report as a valid diagnosis can be made based on a blood count and testing for a genetic mutation. Therefore, cancer registries that rely heavily on pathological data and passive reporting are particularly vulnerable to miss haematological malignancies. From the Nordic population-based cancer registries, all but Sweden have claimed to use hospital discharges to identify potential cancer cases and to send queries to clinicians. Our results and experience from the other Nordic countries support the routine use of external data sources, like the hospital discharge diagnoses or other clinical registers to improve monitoring of particularly haematological malignancies.
We found that only 26.6% of the studied diagnoses were common (found in both) in two independent nationwide healthcare registers. A similar concordance rate of 28% was reported from the British study comparing specialist haematological database to the Thames Cancer Registry in 1994–1996 [Citation16]. Our study confirms the previous finding that both hospital-based registers and population-based cancer registers have deficiencies in collecting and validating data on the incidence of haematological cancers.
In all, 1080 individuals in our material were registered in the FCR but missing in the HILMO. These are most likely true cases but registered only after death in the FCR. These cases could have been clinically diagnosed before 2007 as this data was not extracted from the HILMO for linkage. It is also possible that some of these cases have not received treatment or visited specialist care at all as the natural history of disease is often very long and with unspecific symptoms. Primary healthcare visits are reported to the HILMO gradually since 2012 and were, thus, mostly not available for the study period in hand.
Also HILMO may have deficiencies especially among outpatient visits. We have good reason to believe that a diagnosis found in the HILMO is clinically relevant and correct in the sense of resource usage [Citation7]. However, the HILMO includes erroneous (e.g. preliminary) diagnoses as diagnostic changes are not corrected to earlier notifications. Our estimate of high agreement (0.80 using standard estimate of kappa) is based on patients recorded both in the FCR and in the HILMO. However, both registers had large amount of missing information which is a potential source for bias in the estimation of agreement.
The proportion of microscopically verified cases was 74% in our material. Ending up with a morphological diagnosis (pathology) is dependent upon multiple factors. These are, for instance, patient’s age and compliance to diagnostics, accessibility of the tumour and availability of healthcare services. Thus, the proportion of microscopically verified cases should not reach 100%, as that would indicate missing only clinically diagnosed cases [Citation17]. It is of concern, however, that 24% of the myeloproliferative and myelodysplastic neoplasms were reported based on a death certificate only. This proportion is extremely high compared to approximately 2.5% of annual death certificate registrations among all cancer types in Finland (www.cancer.fi/syoparekisteri/en).
Cancer cases initially identified from death certificates are traced back to the death certifying institution in Finland. However, cause of death is often registered with a long delay from diagnosis and the place of death is not commonly the hospital of diagnosis or treatment. Currently, the national legislation in Finland does not allow use of the HILMO for cancer registry update directly, but we expect a renewal of our legislation in a few years. From 2016 on, we will decrease the high proportion of death certificate registrations among MDSs and MPNs by using hospital discharges to remind clinicians to report the cases.
To our knowledge, this is the first time that the current classification standards and most recent diagnoses of haematological malignancies have been applied to study data quality in a population-based cancer registry. The study was based on comparing two independent nationwide healthcare registers whose data collection is ensured by legislation. Even though administrative register data is a feasible and cost-effective way to study resource use, the data have not been collected for research purposes. As every contact to the healthcare is recorded with some diagnosis code, it is difficult to distinguish incident and prevalent cancer cases from hospital discharge data. This results in over-registration by the HILMO (). Age-standardised incidence rate of MPN and MDS in the HILMO is substantially higher in the beginning and decreases after a few years reflecting more likely the true incidence.
Our results of completeness (47%) should be interpreted as a conservative estimate of these haematological malignancies in the FCR. We wanted to avoid counting morphology codes most likely referring to the same tumour and transformed haematological cancers as incident tumours. Therefore, we only included the first haematological malignancy recorded ever in the FCR. It is possible that some of these patients have MDSs and MPNs registered later on.
Morphology codes used in pathology and haematology laboratories should follow the WHO classification. These and ICD-10 codes used among health service providers should be updated on a regular basis. Cancer registries need to have an access to results of all molecular and genetic analyses related to a malignant case. The same applies for biobanks in the future. Cancer registries are encouraged to use hospital discharge data to supplement missing information on a regular basis. An active trace-back system will most likely increase the coverage of cancers without histological verification and decrease the number of cases registered based on death certificates only.
Supplementary_Table_revision.docx
Download MS Word (16.8 KB)Acknowledgments
We thank doctors at all hospitals and pathology and haematology laboratories and the staff at the Finnish Cancer Registry for their valuable work and collaboration. Special thanks to our Nordic colleagues Elínborg Ólafsdóttir from the Icelandic Cancer Registry, Siri Larønningen from the Cancer Registry of Norway, Terji Petersen from the Faroe Islands Cancer Registry, Maya Søndergaard Milter from the Danish Cancer Registry and Staffan Khan from the Swedish Cancer Register for elaborating the use of hospital discharges in their countries.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gavin A, Rous B, Marcos-Gragera R, Middleton R, Steliarova-Foucher E, Maynadie M, et al. Towards optimal clinical and epidemiological registration of haematological malignancies: Guidelines for recording progressions, transformations and multiple diagnoses. Eur J Cancer 2015;51:1109–22.
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al, editors. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). Geneva: World Health Organization; 2000.
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol 1994;33:365–9.
- Lönnberg S, Leinonen M, Malila N, Anttila A. Validation of histological diagnoses in a national cervical screening register. Acta Oncol 2011;51:37–44.
- Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Geneva: World Health Organization, 2008.
- Sant M, Karjalainen-Lindsberg ML, Maynadié M, Raphaël M, Ferretti S, Giacomin A, editors, et al. Manual for coding and reporting haematological malignancies. Tumori 2010;96.4:i.
- Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 2012;40:505–15.
- Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, et al., editors (2013). Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon: International Agency for Research on Cancer. Available from: http://ci5.iarc.fr.
- Aasbrenn M, Langmark F, Wisløff F. Is registration of multiple myeloma in the Norwegian Cancer Registry good enough? Tidsskr nor Laegeforen 2008;128:2712–4. [Norwegian]
- Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31.
- Nørgaard M, Skriver MV, Gregersen H, Pedersen G, Schønheyder HC, Sørensen HT. The data quality of haematological malignancy ICD-10 diagnoses in a population-based hospital discharge registry. Eur J Cancer Prev 2005;14:201–6.
- Sigurdardottir LG, Jonasson JG, Stefansdottir S, Jonsdottir A, Olafsdottir GH, Olafsdottir EJ, et al. Data quality at the Icelandic Cancer Registry: comparability, validity, timeliness and completeness. Acta Oncol 2012;51:880–9.
- Shi J, Shao ZH, Liu H, Bai J, Cao YR, He GS, et al. Transformation of myelodysplastic syndromes into acute myeloid leukemias. Chin Med J 2004;117:963–7.
- Björkholm M, Derolf AR, Hultcrantz M, Kristinsson SY, Ekstrand C, Goldin LR, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
- Okuyama N, Sperr WR, Kadar K, Bakker S, Szombath G, Handa H, et al. Prognosis of acute myeloid leukemia transformed from myelodysplastic syndromes: a multicenter retrospective study. Leuk Res 2013;37:862–7.
- Phekoo K, Møller H, Richards M, Schey S. Comparison of a specialist haematological malignancy database against a regional cancer registry: case ascertainment and diagnostic accuracy. Br J Haematol 2002;119:697–705.
- Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur J Cancer 2009;45:756–64.