Abstract
Background Breast cancer mortality has declined from 1995 through 2012 which may be attributed to earlier diagnosis, changes in lifestyle risk factors, and improved treatments. To a large extent the relative contribution of these modalities are unknown. Mammography screening was introduced late in Denmark; in 1995 around 20% of the Danish female population aged 50–69 was covered by population-based screening, and this was in 2008 extended to the entire population. Breast conserving surgery gradually replaced mastectomy, and sentinel node biopsy was introduced. In the same period adjuvant treatment was extended considerable.
Methods A population-based study of 68 842 breast cancer patients registered in the clinical database of the Danish Breast Cancer Cooperative Group in 1995–2012. Comprehensive data on prognostic factors, comorbidity and treatment together with complete follow-up for survival were used to evaluate improvements in mortality and standardized mortality rate in successive time periods.
Results The results from this study demonstrated a significant improvement in prognosis in successive time periods covering 1995–2012. Apart from patients with a high Charlson Comorbidity Index (CCI) improvements were seen in all subgroups of patients. Prognostic factors were more favorable in the latest time period accordingly to the introduction of nationwide screening. In the study period adjuvant treatment was extended considerable.
Conclusion The impact of screening was by nature of limited magnitude. The modified treatment strategies implemented by the use of nationwide guidelines seemed to have a major impact on the substantial survival improvements.
The incidence of breast cancer has steadily increased from 1000 patients per year in the early 1950s to around 5000 patients in 2010 corresponding to one of every nine Danish women (NORDCAN www.ancr.nu, [Citation1]). However, breast cancer mortality has declined from 1995 through 2012 which may be attributed to earlier diagnosis, changes in lifestyle risk factors, and improved treatments. To a large extent the relative contribution of these modalities are unknown and it has not been clarified whether the improvements are similar in different subsets of women.
Mammography screening was introduced later in Denmark compared to Northern Europe in general. In 1995 around 20% of the Danish female population aged 50–69 was covered by population-based screening, and this was in 2008 extended to the entire population [Citation2].
Women with breast cancer have successively been exposed to less extensive surgery. Breast conserving surgery (BCS) gradually replaced mastectomy as data emerged from randomized trials and was promoted at the NIH conference in 1991 [Citation3,Citation4]. Smaller margin has been adapted resulting in less tissue removed by BCS and axillary lymph node dissection has in clinically node negative cases been replaced by sentinel node biopsy [Citation5,Citation6]. Increased use of BCS has resulted in a similar increase in the use of radiotherapy, and in addition radiotherapy has increasingly been used in patients with node positive disease following mastectomy [Citation7–10].
Women with early breast cancer are increasingly being exposed to adjuvant systemic therapies, which can safely be omitted in only a small subgroup of node negative patients aged 60 or older with estrogen receptor (ER) positive, HER2 negative, grade I tumors of 10 mm or less [Citation11]. Endocrine treatment is the key component of the adjuvant therapy in patients with ER positive tumors and its duration has gradually been extended [Citation12–14]. Human epidermal growth factor 2 (HER2) is overexpressed or amplified in 10–15% of breast cancers and HER2 positivity is associated with increased tumor aggressiveness, risk of recurrence and breast cancer mortality. Trastuzumab is a monoclonal antibody targeting the extracellular domain of HER2 and was introduced in the adjuvant setting in 2005 following the documentation of a beneficial effect [Citation15,Citation16]. Primarily trastuzumab was offered to patients with invasive cancers >1 cm who otherwise were allocated to chemotherapy, and from 2010 to all patients with a HER2-positive cancer.
Chemotherapy is well established as adjuvant therapy for early breast cancer and the recent overview from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) suggested that on average, chemotherapy reduces 10-year breast cancer mortality by about one third [Citation17]. The proportional benefits were independent of age, nodal status, ER status and type of chemotherapy regimen. Consequently, adjuvant chemotherapy has been increasingly used and is now almost mandatory in patients with ER negative cancers. Chemotherapy has also been introduced to patients with ER positive cancers, but may safely be omitted in about one quarter of postmenopausal ER positive breast cancer patients who following optimal endocrine treatment are free of excess mortality [Citation18].
The aim of the present study was to describe the changes in outcome for Danish breast cancer patients in the period 1995–2012 when mammography screening became nationwide, breast surgery became gentler, and the use of adjuvant systemic therapies was widened. Further to analyze the relative contribution of the changes in outcome attributable to earlier diagnosis and to treatment.
Methods
Since the establishment in 1977 the Danish Breast Cancer Cooperative Group (DBCG) has provided standard diagnostic and treatment algorithms for early breast cancer [Citation19]. Data on diagnostic, therapeutic, and follow-up on newly diagnosed breast cancer patients have been collected prospectively in the DBCG Registry by the use of standardized forms. A population of all patients diagnosed in the period 1995–2012 were included in the present analysis. Data from the Danish National Patient Register (NPR) and the Central Population Register (CPR) were linked to the DBCG database using the unique personal identification number assigned to all Danish citizens by the CPR. The NPR has collected data on a nationwide basis on all somatic hospital admissions since 1977 and data on outpatients and emergency patients since 1995. NPR includes, on an individual level, information from all hospitalizations, including dates of admission and discharge and up to 20 discharge diagnoses per hospitalization. The CPR holds information on vital and emigration status on all Danish citizens. A complete follow-up for survival was achieved for 68 550 patients (99.6%) while 292 patients emigrated up to 18 years and 6 months after surgery. The estimated potential median observation time was 9 years and 9 months.
Comorbidity
Comorbidity was described according to Charlson Comorbidity Index (CCI) [Citation20]. The CCI is a weighted index that takes into account both the number and the seriousness of 19 chronic conditions. The CCI in the present study was based on hospital contacts using International Classification of Diseases ICD-8 and ICD-10 data up to 10 years prior to the breast cancer diagnosis date.
Statistical analysis
Follow-up time was quantified in terms of a Kaplan-Meier estimate of potential follow-up. Time at risk was defined as time from diagnosis until date of death from any cause, emigration, or end of follow-up. Univariate and multivariate Poisson regression analysis for mortality and standardized mortality ratio (SMR) were used. The SMR, computed as the ratio of the observed to the expected number of deaths, served as an estimate of relative risk of death. The number of deaths expected was calculated by applying age- and calendar year-specific female mortality figures of the general Danish population and the corresponding person-years of follow-up for the respective cohort members. Ninety-five percent confidence intervals (CI) were computed based on the assumption that the observed number of deaths followed a Poisson distribution. The regression models were used to assess the relative risk (RR) within subgroups. Factors included in the multivariable analyzes were year of diagnosis, age, CCI, extension, tumor size, lymph node status, ER status, histological type and grade, and lymphovascular invasion. ER status was included with a time-dependent component. Interactions between covariates were investigated in separate models by applying the Wald test in the multivariate models.
Three outcome measures relating to year one, five and 10 after diagnosis have been calculated: absolute all-cause mortality rate per 100 person-years, absolute overall survival, and relative survival. All estimates were age-standardized according to the International Cancer Survival Standard (ICSS) 1 population. Absolute all-cause mortality rate (reported per 100 person-years) was calculated as the number of deaths divided by the sum of the patient-time at risk during the period concerned. Relative survival was estimated as the ratio of the observed survival of the patients (all deaths considered) to the expected survival. The expected survival was estimated from the general Danish female population, matched by age and calendar time. χ2-test statistics were used to evaluate heterogeneity between the calendar periods. Statistical analyses were performed with the STATA v11.0 (StataCorp, College Station, TX, USA) and SAS v9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
The clinical DBCG database included 68 842 patients diagnosed with invasive breast cancer between 1995 and 2012 (). Overall, the disease was operable at diagnosis in 90% of patients, locally advanced in 1.4%, with distant metastasis in 1.9% and loco-regional extend was unknown in 7.8%.
Table 1. Patient characteristics.
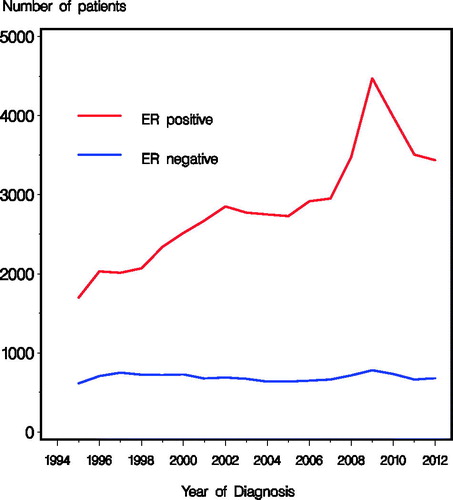
summarizes patient and tumor characteristics according to treatment period (1995–1999, 2000–2004, 2005–2009, and 2010–2012) in the 61 166 patients who initiated therapy with a curative intent and underwent surgery. Reflecting the increasing incidence the number of patients increased from 2578 in 1995 to 3760 in 2012. A peak in incidence was observed among women age 50–69 following the implementation of the national screening program in 2008. During the periods, substantial and statistical significant changes were observed in tumor characteristics including an increasing proportion of tumors sized 0–10 mm (p < 0.0001) and a decreasing proportion with four or more positive nodes (p < 0.0001) (). The number of patients with ER negative tumors was constant through the time periods, whereas the ER unknown significantly decreased from the first period, and the number of patients with ER positive tumors increased steadily with a peak for 2008–2010 (, ).
Table 2. Patient characteristics among patients with early breast cancer and known nodal status.
shows the number of patients who within each period received available loco-regional and systemic therapies. The predominant primary type of surgery switched completely from mastectomy (77%) to BCS (67%) during the study period. Among patients with mastectomy, the proportion receiving radiotherapy increased significantly in the second period, and then declined slowly. A similar pattern was seen regarding extending radiotherapy to regional nodes after lumpectomy. The use of systemic therapy increased significantly; the proportion of patients where systemic treatment were unknown dropped from 24% to 9%, and of the remaining patients, the proportion who did receive systemic therapy increased from 49% to 88%, comparing 1995–1999 with 2010–2012. An increase in the proportion of patients who received chemotherapy in addition to targeted therapy from 7% to 43% was seen. About half of those who received chemotherapy had a taxane-based regimen. In 80% of postmenopausal patients endocrine therapy included an aromatase inhibitor.
Table 3. Treatment characteristics among patients with early breast cancer and known nodal status.
Using 1995–1999 as reference group, highly significant improvements were observed in the succeeding periods (, , ) regarding mortality and SMR. The estimates for each time period improved adjusting for comorbidity at time of breast cancer diagnosis and further when also tumor extension was accounted (). Similar developments with even more distinct enhancements were seen when the results were restricted to the group of patients with early breast cancer (). The same pattern was seen for both mortality and SMR, although less pronounced for SMR () reflecting the general improvements in survival in the Danish population. Adjustment for tumor characteristics slightly reduced the improvements ascribed time periods ().
Figure 2. Relative risk of mortality (A and B) and SMR (C and D) according to calendar time, including all patients (A and C) and patients with early breast cancer and known nodal status (B and D). *Adjusted for age, CCI, tumor size, nodal status, histological type and grade, lymphovascular invasion and estrogen receptor status.
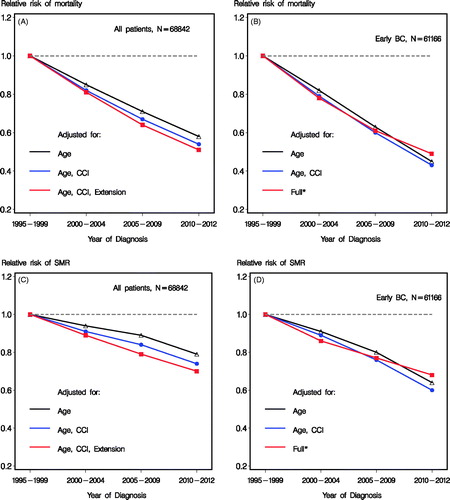
Table 4. Overall survival (95% CI).
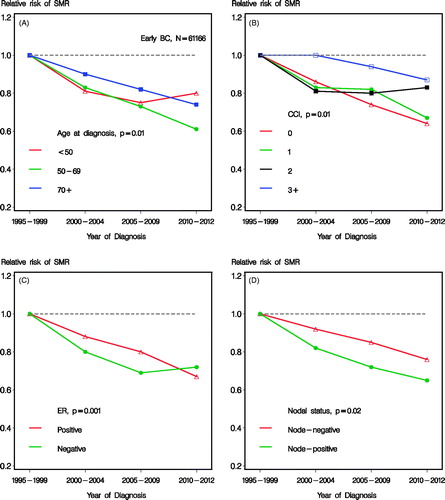
shows the heterogeneity of improvement in mortality over time according to subgroups of age, CCI, ER status, and nodal status, respectively. Test for heterogeneity are shown. High age () and high comorbidity score () showed a less pronounced improvement, although still statistical significant (p < 0.0001). Also ER status and nodal status showed statistical significant heterogeneity for improvement over time (). No statistical significant heterogeneity according to tumor size (0–20 mm vs. 21 + mm) was seen (p = 0.34, data not shown). The analogous improvements in SMR over successive time periods are shown in , where similar heterogeneity according to subgroups was seen. The improvement seen in mortality for the last time period and the youngest age group were modest (), translating to estimates of RR relating to SMR of 0.75 (95% CI 0.67; 0.84) for 2005–2009 and 0.80 (95% CI 0.66; 0.96) for 2010–2012, not being statistically different (). Patients with comorbidity score 3 + did not obtain a significant (p = 0.58) prognostic improvement (), although a trend of improvement was observed, with an estimate of 0.87 (95% CI 0.69; 1.10) for 2010–2012 compared to 1995–1999.
Figure 3. Relative risk of mortality according to calendar time and subgroups of age (A), CCI (B), estrogen receptor (ER) status (C), and nodal status (D), respectively. Including patients with early breast cancer and known nodal status and adjusted for age, CCI, tumor size, nodal status, histological type and grade, lymphovascular invasion and estrogen receptor status.
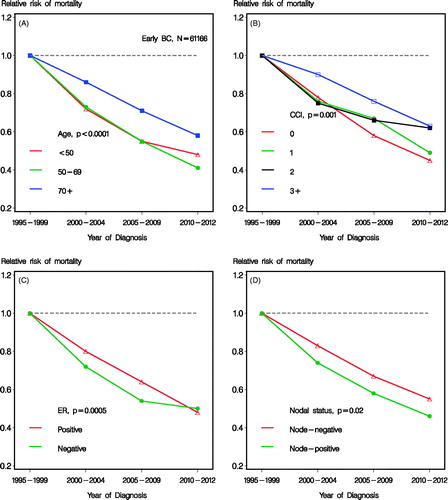
Figure 4. Relative risk of SMR according to calendar time and subgroups of age (A), CCI (B), estrogen receptor (ER) status (C), and nodal status (D), respectively. Including patients with early breast cancer and known nodal status and adjusted for age, CCI, tumor size, nodal status, histological type and grade, lymphovascular invasion and estrogen receptor status.
Discussion
The results presented show a significant improvement of the prognosis in breast cancer patients diagnosed in the period 1995–2012. Improvements were shown adjusted for of age, presence of comorbidities, and whether or not patients were considered operable at primary diagnosis. For patients with operable breast cancer improvements were shown irrespective of tumor size, lymph node status, and ER status. The improvement in SMR were 0.69 (95% CI 0.64; 0.74) comparing 2010–2012 with 1995–1999, and in the same period adjuvant treatment was extended considerable. Young age (≤35) was in 2002 included as an independent factor for allocation to systemic treatment, and gradually the low-risk group not having systemic treatment were raised and reached 60 years in 2010. For patients younger than 50 only a modest decrease in RR of mortality was obtained from 2005–2009 to 2010–2012 reflecting that treatment was not further improved beyond taxanes and trastuzumab. ER negative status was implemented as an independent factor for allocation to systemic treatment around 2000, and hereafter a marked improvement in outcome was seen compared to the previous period. The introduction of the sentinel node technique after 2001 resulted in stage migration with the potential of improved prognosis in both nodal groups.
As a result of accumulating evidence and international consensus the use of adjuvant treatment increased significantly and the close linkage to the successive survival improvements, even with adjustment for changes in prognostic factors, supported a causal relationship.
Patients with a high comorbidity score did not achieve a statistical significant prognostic improvement, and this may in part be explained by reluctance towards adjuvant treatment in this group. Although improvements were observed in all age groups, patients 70 years and older obtained relatively less benefit which may have several explanations. Although the use of adjuvant treatment increased with time, patients 70 years or older were in contrast to younger patients not allocated to regional radiotherapy if node positive or to chemotherapy irrespective of other risk factors if ER negative in the 1990s and the beginning of the 2000s. The presence of comorbid conditions was significantly higher among older patients, and this may cause a restraint in applying optimal treatment regimens [Citation21].
The proportion of patients with ER positive disease steadily increased during the study period [Citation22]. In addition, the extension of screening covering the entire female population aged 50–69 years had an impact on prognostic factors, and especially a pronounced peak in incidence of ER positive cancer was seen. The improved prognosis was partly explained by these changes. This was apparent comparing estimates of improvements over time adjusted for age and CCI alone with estimates adjusted also for prognostic factors including tumor size, nodal status and hormone receptor status. The distinction was evident in the latest time period, when the changes in prognostic factors were observed. Thus, mammography screening had some influence on the observed survival improvements. Although the peak in ER positive patients observed with the introduction of nationwide screening partly explain the better prognosis overall, it does not solely explain a better outcome, as clear improvements were documented in the ER positive only subgroup. With this short time frame the impact of screening is limited to the age group 50–69, which constitutes just above half of the entire population.
The CISNET consortium reported the impact of screening and adjuvant treatment as modeled using SEER data by seven different research groups [Citation23]. The models consistently showed that the observed mortality data could not be explained by screening or adjuvant treatment alone. The relative improvements ascribed to screening and adjuvant treatment however varied considerable across models. Another limitation was that in the models the options for adjuvant treatment were restricted to tamoxifen and/or chemotherapy and the possible benefit of these treatments were prespecified according to age, year, stage and hormone receptor status. Two additional studies using SEER data attempted to analyze the relative contribution to improvement in breast cancer survival of screening and adjuvant treatment [Citation24,Citation25]. By evaluating secular trends in tumor size and ER status, these studies reversely concluded that the relative contribution of screening and adjuvant treatment were, respectively, the key component of the substantial improvements presented.
Several aspects should be considered when interpreting the present study. The large size and population-based design of our nationwide clinical database in an environment with free access to public healthcare minimized the potential risk of selection bias. Adjuvant treatment was registered in more than 90% of the patients in the last two periods but was unknown in a fourth of the patients in the first period. However, independent of time period the available data on adjuvant therapy are comprehensive and comparable to that of a clinical trial. Patients were included irrespective of availability of data on adjuvant treatment and linkage to the NPR and CPR registries ensured a complete follow-up for mortality and comorbidity in the entire population.
For the patient cohort presented in this study, the impact of screening was by nature very limited. Estimates for only one time period after the implementation of nationwide screening were available. The possibility of presenting data both before and after implementation of nationwide screening was a clear strength. The use of nationwide guidelines for treatment strategies together with a registration of the adherence hereof was obviously an advantage for the interpretation of the result presented. The results clearly demonstrated a significant improvement in prognosis in successive time periods covering 1995–2012. Apart from patients with a high CCI improvements were seen in all subgroups of patients. Except for CCI, prognostic factors were more favorable in the latest time period, and accordingly the impact of screening was obvious, although of limited magnitude. The successive modifications of treatment strategies implemented by the use of nationwide guidelines seemed to have a major impact.
Supplementary Table 1. Mortality per 100 person-years [95%CI].
Supplementary Table 2. Relative Survival [95% CI].
Supplementary Table 3. Relative risk (95% CI) of mortality (A and B) and SMR (C and D) according to calendar time, including all patients (N = 68,842, A and C) and patients with early breast cancer and known nodal status (N = 61,166, B and D). See Figure 2.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Engholm G , Ferlay J , Christensen N , Bray F , Gjerstorff ML , Klint A , et al . NORDCAN-a Nordic tool for cancer information, planning, quality control and research. Acta Oncol 2010; 49: 725– 36.
- Christiansen P , Vejborg I , Kroman N , Holten I , Garne JP , Vedsted P , et al . Position paper: Breast cancer screening, diagnosis, and treatment in Denmark. Acta Oncol 2014; 53: 433– 44.
- Blichert-Toft M , Nielsen M , Düring M , Møller S , Rank F , Overgaard M , et al . Long-term results of breast conserving surgery versus mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG- 82TM protocol. Acta Oncol 2008; 47: 672– 81.
- NIH consensus conference. Treatment of early-stage breast cancer. JAMA 1991; 265: 391– 5.
- Tvedskov TF , Jensen MB , Balslev E , Ejlertsen B , Kroman N. Stage migration after introduction of sentinel lymph node dissection in breast cancer treatment in Denmark: A nationwide study. Eur J Cancer 2011; 47: 872– 8.
- Houssami N , Macaskill P , Marinovich ML , Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: A meta-analysis. Ann Surg Oncol 2014; 21: 717– 30.
- Overgaard M , Hansen PS , Overgaard J , Rose C , Andersson M , Bach F , et al . Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997; 337: 949– 55.
- Overgaard M , Jensen MB , Overgaard J , Hansen PS , Rose C , Andersson M , et al . Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641– 8.
- Darby S , Mcgale P , Correa C , Taylor C , Arriagada R , Clarke M , et al . Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15- year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378: 1707– 16.
- Mcgale P , Taylor C , Correa C , Cutter D , Duane F , Ewertz M , et al . Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383: 2127– 35.
- Christiansen P , Bjerre K , Ejlertsen B , Jensen MB , Rasmussen BB , Lænkholm AV , et al . Mortality rates among early-stage hormone receptor-positive breast cancer patients: A population-based cohort study in Denmark. J Natl Cancer Inst 2011; 103: 1363– 72.
- Davies C , Godwin J , Gray R , Clarke M , Cutter D , Darby S , et al . Early Breast Cancer Trialists Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771– 84.
- Davies C , Pan H , Godwin J , Gray R , Arriagada R , Raina V , et al . Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381: 805– 16.
- Dowsett M , Forbes JF , Bradley R , Ingle J , et al . Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341– 52.
- Piccart-Gebhart MJ , Procter M , Leyland-Jones B , Goldhirsch A , Untch M , Smith I , et al . Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659– 72.
- Perez EA , Romond EH , Suman VJ , Jeong J-H , Sledge G , Geyer CE , et al . Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J Clin Oncol 2014; 32: 3744– 52.
- Peto R , Davies C , Godwin J , Gray R , Pan HC , Clarke M , et al . Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 2012; 379: 432– 44.
- Ejlertsen B , Jensen M-B , Mouridsen HT. Excess mortality in postmenopausal high-risk women who only receive adjuvant endocrine therapy for estrogen receptor positive breast cancer. Acta Oncol 2014; 53: 174– 85.
- Møller S , Jensen M-B , Ejlertsen B , Bjerre KD , Larsen M , Hansen HB , et al . The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008; 47: 506– 24.
- Charlson ME , Pompei P , Ales KL , Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373– 83.
- Land LH , Dalton SO , Jensen M-B , Ewertz M. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer 2012; 107: 1901– 7.
- Anderson WF , Rosenberg PS , Petito L , Katki Ha , Ejlertsen B , Ewertz M , et al . Divergent estrogen receptor-positive and -negative breast cancer trends and etiologic heterogeneity in Denmark. Int J Cancer 2013; 133: 2201– 6.
- Berry D , Cronin K , Plevritis SK , Fryback DG , Clarke L , Zelen M , et al . Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784– 92.
- Elkin EB , Hudis C , Begg CB , Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975-1999. Cancer 2005; 104: 1149– 57.
- Park J , Anderson WF , Gail MH. Improvements in US Breast Cancer Survival and Proportion Explained by Tumor Size and Estrogen-Receptor Status. J Clin Oncol 2015; 33: 2870– 6.