Abstract
Background Solid organ transplant recipients are at increased risk of developing malignancies. The objective of this prospective, observational, one-armed study was to study the feasibility to add a mammalian target of rapamycin (mTOR) inhibitor to the immunosuppressive regimen in transplanted patients with post-transplant malignancies. During the trial the need to improve identification of post-transplant malignancies and to reassure adequate oncological treatment of these patients became evident. Multidisciplinary team (MDT) evaluation of oncological and immunosuppressive treatments was implemented for all patients with malignancies after renal or combined renal and pancreas transplantation because of the trial.
Material and methods At Uppsala University Hospital, Sweden, a MDT consisting of transplant surgeons, nephrologists, oncologists and dermatologists evaluated 120 renal or combined renal and pancreas-transplanted recipients diagnosed with malignancies from September 2006 to July 2012. To identify all malignancies, the population was linked to the Regional Tumor Registry (RTR). We recorded to which extent a switch to mTOR inhibitors was possible and how often the originally planned oncological managements were adjusted. All patients were followed for three years. (ClinicalTrials.gov: NCT02241564).
Results In 76 of 120 patients (63%) a switch to mTOR inhibitors was possible. Immunosuppression was interrupted in seven patients (6%), reduced in three patients (2%) and remained unchanged in 34 of 120 patients (28%). Identification of post-transplant malignancies increased significantly after linkage to RTR (p = 0.015). The initially recommended oncological treatment was adjusted in 23 of 44 patients (52%) with solid or hematological malignancies; 36 of these patients (82%) were treated according to national guidelines.
Conclusion In two thirds of the patients the immunosuppressive treatment could be changed to an mTOR inhibitor with anti-tumor effects in transplanted patients with post-transplant malignancies. The use of regional tumor registers considerably improved the identification of patients with post-transplant malignancies indicating that post-transplant malignancies might be timely underreported in transplant registers.
The incidence of malignant tumors after renal transplantation is elevated 2–5-fold in comparison to the general population [Citation1–5]. This is mainly caused by the continuous use of immunosuppression to prevent graft rejection [Citation6,Citation7]. More than 90% of the different tumors post-transplantation occur at increased rates [Citation3,Citation8], especially those of known or suspected viral origin [Citation4,Citation5,Citation8]. Register studies of the transplanted population have shown that even if the ratio of post-transplant malignancies is elevated, the number of patients with a defined cancer diagnosis is limited due to the high diversity of cancer types [Citation4]. Accordingly, only clinical studies regarding post-transplant lymphoid disorder and skin cancer, but not solid cancer, have been conducted.
Post-transplant malignancies are associated with high morbidity and mortality [Citation5] and these tumors are considered more aggressive and are associated with worse outcome compared to those of the general population [Citation6,Citation9–11]. Extended screening of solid tumors in the renal transplanted population is, however, not recommended due to low cost effectiveness, but regular skin screening is advisable [Citation12].
In patients with post-transplant malignancies the immunosuppression is generally reduced to prevent further growth and spread of malignant cells [Citation6,Citation7]. Furthermore, the immunosuppressive effects of chemotherapy occasionally render immunosuppression unnecessary. A switch from calcineurin inhibitors (CNI) to mammalian target of rapamycin (mTOR) inhibitors could be considered as mTOR inhibitors have both immunosuppressive and anti-tumor properties [Citation6,Citation7,Citation13–15]. mTOR inhibitors also enhance radiation therapy with approximately 10% [Citation16,Citation17].
Multidisciplinary cancer conferences contribute to improved clinical decision making, clinical outcome and patient experience in the general population [Citation18]. Comorbidity, such as renal dysfunction, is a significant negative prognostic factor for long-term cancer survival and may limit the patients’ access to adequate cancer treatments. At multidisciplinary conferences, treatment suggestions for patients with comorbidity tend not to follow clinical guidelines [Citation19]. Accordingly many cancer patients with comorbidity, such as solid organ transplantation, receive more conservative treatment despite the fact that an active treatment most of the time probably would be tolerated [Citation19].
The objective of this prospective clinical observational study was to investigate the possibility to change to or add an mTOR inhibitor to the immunosuppressive regimen for patients with post-transplant malignancies, i.e. in patients with a defined comorbidity. We also wish to communicate our work to timely improve identification of post-transplant malignancies.
Material and methods
Patient cohort
Uppsala University Hospital serves a population of two million inhabitants. Between June 1969 and December 2012, 2601 renal or simultaneous renal and pancreas transplantations were performed in 2196 patients. A total of 531 patients were diagnosed with malignancies; 117 were diagnosed with pre-transplant malignancies and 414 patients with post-transplant malignancies. All patients who had encountered a malignancy or recurrence of malignancy from September 2006 or at most one year before inclusion were eligible to be included in this study (n = 230). The inclusion criteria were: patients >18 years willing and capable of giving written informed consent of participation in the project and with a previous or present malignancy (other than basal cell carcinoma). All participants signed a written informed consent. One hundred and twenty study patients were included between September 2007 and December 2012. Follow-up data until June 2015 were included. This study was approved by the Regional Ethical Review Board in Uppsala (Dnr 2007/032). The study “Malignancies in transplanted patients (MALTX)” was registered at ClinicalTrials.gov (Identifier: NCT02241564).
Sources of cancer information
Information, including data about malignancies, from transplant recipients is collected annually and registered in the University Hospital Transplantation Database. Since January 1982 the findings are also reported to the Collaborative Transplant Study (CTS) Heidelberg, the world’s largest transplantation register with more than 400 000 organ transplantations registered [Citation20].
The Swedish Cancer Register covers the entire Swedish population and the Regional Tumor Register (RTR) of the Uppsala-Örebro region includes the tumors of our transplanted patients. More than 98% of all malignancies are reported and 97% of the histological verification can be found in the register.
The patients in the study were initially identified in the University Hospital Transplantation Database or by referral from the local nephrologists. Since 2009, malignancy information was retrieved from RTR, linked to the Transplantation Database three to four times per year. The cancer incidence, before and after linkage to RTR, was compared to other European countries reporting to CTS.
Study design
The study was designed as a prospective, observational one-armed study. A multidisciplinary team consisting of transplant surgeons, nephrologists, oncologists and dermatologists evaluated all patients regarding the oncological and immunosuppressive treatment. This included the aim to add or to convert to mTOR inhibitors. In this study everolimus (Certican; Novartis Pharma AG, Basel, Switzerland) was used as mTOR inhibitor. Patients with the following criteria were eligible for an addition of or switch to everolimus: Patients with a GFR >20 ml/min, hemoglobin count >80 g/dl, platelet count >50 × 109/l, white blood cell count >2.5 × 109/l, total cholesterol <9 mmol/l, triglycerides <6 mmol/l, spot urinary albumin/creatinine ratio <70 mg/mmol and transaminases or bilirubin within normal ranges. Everolimus was given in combination with low dose CNI (see trough levels below) in patients at higher immunological risk defined as >30% panel reactive antibodies, patients with previous episodes of acute rejections or retransplanted patients, and in patients with adverse events on full dose of mTOR inhibitors. A total switch from CNI to everolimus was performed in patients with <30% panel reactive antibodies. In practice, the change in immunosuppression was performed over one day at the time of inclusion.
Changes of immunosuppression, adjustment of the oncological treatment, tumor progression, regression or reoccurrence of the diagnosed malignancy, de novo diagnosed malignant tumors, renal function, graft and patient survival and immune response were followed. The trough levels of different immunosuppressants after adding everolimus (low dose) to the treatment regimen were 4–6 ng/ml for everolimus, 30–80 ng/ml for cyclosporine A and 1.5–4 ng/ml for tacrolimus. The trough level of everolimus (full dose) was 6–8 ng/ml after switching from cyclosporine A or tacrolimus.
All patients were examined at the first visit, 2–3 months after inclusion, and once yearly until three years after inclusion.
Follow-up information about patients who were unable to appear at regular visits was collected from the regional hospitals.
ImmuKnow assay
The ImmuKnow Immune Cell Function Assay (Cylex, Inc., Columbia, MD, USA) was used to follow cell-mediated immunity [Citation21].
Statistical methods
The post-transplant tumor incidence was determined by using the Kaplan-Meier (KM) analysis. The Log-Rank (Mantel-Cox) test was used within a 95% confidence interval to determine whether the KM survival curves were statistically different between the renal transplanted population in Uppsala and the renal transplanted population in CTS before and after the linkage to RTR. The significance levels were adjusted for multiple testing by Bonferroni’s corrections. SPSS version 22 statistical software (IBM, Armonk, NY, USA) was used for all KM analyses. Tumor incidence was calculated as standardized incidence ratio (SIR) with expected and observed counts. The renal transplanted population in CTS was considered “expected” and the renal transplanted population in Uppsala was considered “observed”.
ImmuKnow and serum creatinine levels were presented as mean ± standard deviation (SD). The non-parametric Kruskal-Wallis test was used to evaluate the difference in ImmuKnow and serum creatinine levels from the time of inclusion until the three-year follow-up. A p-value less than 0.05 was considered significant.
Results
Inclusion and exclusion
In total 230 patients were diagnosed with post-transplant malignancies. One hundred and ten patients were not enrolled due to lack of information of the malignancy at the Department of Transplantation before the linkage to RTR (n = 85), poor general condition (n = 18) or no wish to participate (n = 7) ().
Figure 1. CONSORT flow diagram of a prospective, clinical, non-randomized, one-armed observational study of post-transplant malignancies in renal transplant patients. RTR, Regional Tumor Registry.
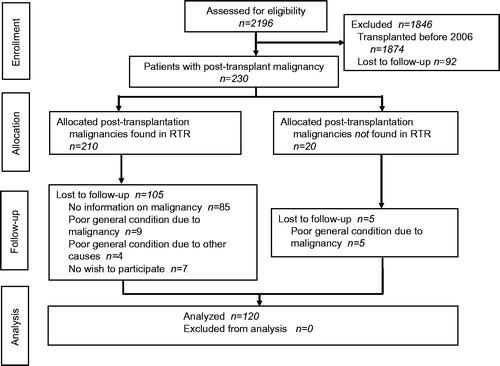
One hundred and eight study patients (90%) were registered in RTR. Information of the remaining 12 patients (10%) was obtained from the regional hospitals. (The corresponding registration rate in RTR for the general population is estimated to be 98%. Only around 10% of all malignancies are reported with a delay of >3 months).
Characteristics of the included patients
Demographic characteristics of the 120 study patients and malignancy data are presented in .
Table 1. Characteristics of patients and malignancies at inclusion.
Change in immunosuppression
In total, the immunosuppression was converted to everolimus either by a complete switch (n = 44) or as an addition to low dose CNI (n = 32) for 76 of 120 patients (63%) (). In 44 patients no mTOR inhibitor was given according to the following reasons: The total immunosuppression was interrupted in seven patients (6%), reduced in three patients (2%) and was unchanged in 34 of 120 patients (28%), due to contraindications (n = 21), dialysis (n = 8), non-compliance (n = 3), poor general condition (n = 1), interactions between chemotherapy and immunosuppression (n = 3) and no wish to change immunosuppression (n = 8). Among patients with solid or hematological malignancies the immunosuppression was switched to mTOR inhibitors in 35 of 44 patients (80%), interrupted in six patients (14%) and reduced in one patient (2%). Thus only two of 44 patients continued with their original immunosuppression. Among patients with skin malignancies, the immunosuppression was switched to mTOR inhibitors in 40 of 76 patients (53%), interrupted in one patient and reduced in two patients. Thus 33 of 76 patients (43%) continued with their original immunosuppression (). Within one year 28 of 76 patients (37%) with mTOR inhibitors interrupted the treatment due to adverse effects ().
Table 2. Changes in immunosuppressive treatment.
Table 3. Adverse events due to mTOR inhibitors leading to discontinuation of the drug.
Patient identification and incidence of malignancies post-transplantation
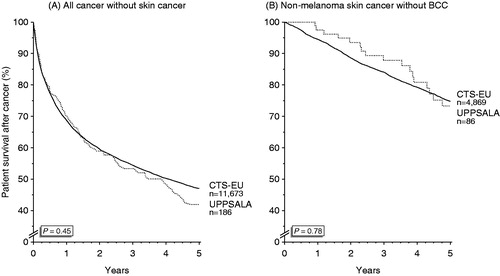
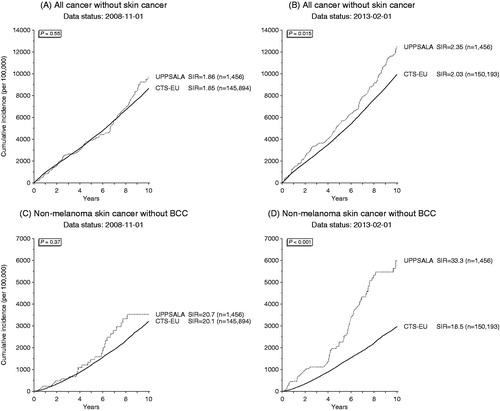
When the project started, the cumulative incidence of post-transplant malignancies in Uppsala was equal to other EU countries reporting to CTS with a SIR of 1.86 versus 1.85 for solid organ and hematological malignancies () (p = 0.55) and an SIR of 20.7 versus 20.1 for skin malignancies () (p = 0.37). From January 2009, when linkage of the transplanted patients with RTR was started, the cumulative incidence of all malignancies increased compared to other EU countries with an SIR of 2.35 versus 2.03 for solid organ and hematological malignancies () (p = 0.015) and an SIR of 33.3 versus 18.5 for skin malignancies () (p < 0.001). All types of tumors, but especially more skin and gynecologic malignancies were detected. The rate of mortality from malignancy was compared between Uppsala and the other EU countries to determine whether this is due to diagnosis of less advanced malignancies, such as in situ cancers. The mortality rate was similar in Uppsala and the other EU countries when looking at all post-transplant malignancies () (p = 0.45) or isolated non-melanoma skin cancer () (p = 0.78).
Figure 2. Cumulative incidence of post-transplant malignancies except skin cancer (A and B) and non-melanoma skin cancer (C and D) in Uppsala, Sweden (dotted line) and the European countries (black) before (A and C) and after (B and D) linking the transplanted patients at Uppsala University Hospital with the Regional Tumor Registry.
Characteristics of solid organ and hematological malignancies
The 44 patients with solid organ or hematological malignancies represented a multitude of diagnoses (Supplement 1, available online at http://www.informahealthcare.com). Six patients had a history of malignancy before the first transplantation (1–25 years). For five of them the previous malignancy differed from the current one. At inclusion of this study, 26 patients had localized malignancy and 18 patients were diagnosed with metastatic malignancy. Five patients were diagnosed with hematological malignancies.
Characteristics of skin malignancies
Three patients suffered from malignant melanoma and 73 from SCC. Among the patients with SCC, 37 (51%) were diagnosed with SCC in situ and 36 (49%) with invasive SCC.
Oncological treatment of solid or hematological malignancies
At the MDT conferences the planned chemotherapy was modified in 19 of 44 patients (43%), the planned radiation therapy was adjusted in 10 of 44 patients (23%) and/or the planned surgical treatment was altered in four of 44 patients (11%) ( and Supplement 1). In total, the oncological treatment was changed in 23 of 44 patients (52%). As a result of the MDT decisions 36 of 44 patients (82%) received adequate oncological treatment in line with the national guidelines, despite renal dysfunction and immunosuppression (Supplement 1). Less than 20% were not treated in compliance with the guidelines. For these patients the oncological therapy was reduced or changed due to renal dysfunction, poor general condition or the patient’s choice.
Table 4. Oncological treatment.
To sum up, the MDT conferences modified the oncological and/or immunosuppressive treatment for 43 of 44 patients with solid or hematological malignancies.
The time between malignancy diagnosis and enrollment did not differ between the patients with adjusted or not adjusted oncological treatment.
Treatment of skin malignancies
All malignancies of the skin were treated by the time the patients were enrolled in the study. Consequently, the MDT did not suggest any oncological treatment modifications of the skin malignancies.
Outcome for patients with solid organ or hematological malignancy
During the first year of follow-up 18 patients with solid or hematological malignancy remained in complete remission and four patients remained in partial remission. Six patients were considered to have stable disease and five patients progressed. One patient developed de novo malignancy and nine patients died. One patient was lost to follow-up.
Twenty-nine of the 44 patients with solid organ or hematological malignancies were followed for three years. Of these, 14 patients remained in complete remission. One patient was considered to have stable disease. Two patients progressed and two patients developed de novo malignancies. Ten patients died of cancer-related death. Five patients were lost to follow-up.
Outcome for patients with skin malignancy
Seventy of the 73 patients with non-melanoma skin cancer were followed for three years. Of these 14 patients developed de novo non-melanoma skin cancer between one and three years of follow-up and nine patients died.
Of the three patients with malignant melanoma one developed a de novo non-melanoma skin cancer within three years of follow-up.
Renal function, patient and graft survival
Serum creatinine was mainly unchanged during the follow-up for all patients with a functioning graft at inclusion (p = 0.644).
The overall one-year patient survival was 90%. Nine patients with solid organ or hematological malignancy died during the first year of follow-up. Thus one-year patient survival of these patients was 79%. Three patients with skin malignancies died during the first year of follow-up, i.e. patients with skin malignancy had a one-year patient survival of 96%. All deaths except two were due to malignancies.
Between one and three years of follow-up 11 patients of 34 with solid or hematological malignancy and nine of 71 patients with non-melanoma skin cancer died. All patients with malignant melanoma were alive after three years. Six patients were lost to follow-up among patients with, one during the first year of follow-up and five more patients during the three years of follow-up. Six patients were lost to follow-up among skin malignancy patients, two during the first year of follow-up and four during the next years. The overall three-year patient survival was 70%. The reasons for lost to follow-up were that the patients were too ill to participate in the study or that they did not want to participate.
Nine patients had turned to dialysis before inclusion with three patients in the solid organ or hematological malignancy group and six in the skin malignancy group. During the first year of follow-up three grafts were lost, all among the skin cancer patients. The overall one-year death censored graft survival was 89%. For patients with solid or hematological malignancies it was 91% and for patients with skin malignancies it was 87%. Between one and three years of follow-up eight more grafts were lost, five grafts among patients with solid or hematological malignancies and three grafts among patients with skin malignancies. Among the three patients with malignant melanoma one was on hemodialysis at inclusion, the two other patients had functioning graft throughout the study. The overall three-year death censored graft survival was 74%.
Cell-mediated immunity during immunosuppressive treatment
No change in ImmuKnow level was observed at group level between baseline (324 ± 128 ng/ml) and three-year follow-up (361 ± 161 ng/ml) (p = 0.358).
Discussion
This is, to our knowledge, the first prospective study on consecutive transplanted patients with solid organ, hematological or skin malignancies with systematic MDT conferences for these patients. An addition of or switch to mTOR inhibitors in post-transplant malignancies may be desirable, as it has a theoretical effect on 90% of all tumor types [Citation22]. This decision making of the immunosuppressive regimen appears as an important topic for post-transplant MDTs [Citation23,Citation24]. Equally important is to timely find these patients as depicted by our struggle to identify them early in different registers.
In these patients immunosuppression was possible to modify in 71% of the patients with post-transplant malignancies, and the addition or switch to mTOR inhibitors was possible for 63% of all patients, (in 53% of the patients with skin malignancy and in 80% of the patients with solid or hematological malignancy). It appeared easier to change the immunosuppression in patients with solid organ or hematological malignancies compared to patients with malignancies of the skin. As shown in other studies mTOR inhibitors were moderately tolerated as one third of the switched patients had to switch back to their original immunosuppression due to adverse effects. In this and other transplant studies pneumonitis occurs in 10%, while the corresponding figure in oncological surveys reaches 30% [Citation25]. This is probably due to higher exposure of mTOR inhibitors when used as anticancer drug alone.
Oncological treatment in combination with or without mTOR inhibitors resulted in stable disease, partial or complete remission in 64% of the patients with solid or hematological malignancies at one year of follow-up and in 48% of the patients at three years of follow-up. This relative contribution of the adjusted oncological treatment and the switch to mTOR inhibitors, is however, somewhat difficult to interpret as the included patients represented a multitude of malignancy diagnosis. mTOR inhibitors were given together with low dose CNI in patients at higher immunological risk defined as immunized patients, patients with previous episodes of acute rejections or retransplanted patients, and in patients with adverse events on full dose of mTOR inhibitors. The change to mTOR inhibitors was safe regarding kidney outcome and the ImmuKnow measurements indicated that cell-mediated immunity was unaltered during immunosuppressive treatment.
Patients with post-transplant malignancies constitute a very heterogeneous population in terms of graft function and type and stage of the malignancy [Citation6]. There are only limited numbers of patients with a defined diagnosis, and this is the reason why we performed a one-armed observational study. The lack of a control group is one of the weaknesses of this study but it was not possible to find a representative control group. The main question remains, how do you design a valid randomized study in these patients.
The CTS Registry in Heidelberg is one of the largest transplant registries collaborating with more than 300 transplant centers worldwide. The risk of underreporting malignancies has been managed by excluding patients from CTS cancer analysis if the annual confirmation regarding malignancies from the participating centers was missing. [Citation26]. Post-transplant incidence of malignancies in Uppsala was initially shown to be at the same level as in other European countries according to the CTS registry. After linkage between the Hospital Transplantation Database and RTR the incidence of post-transplant malignancies in our region was significantly higher in comparison to other European countries. This suggests that malignant diseases generally are underreported in transplant registries. A prerequisite for this strategy is that the patients with cancer are identified. Different strategies to accomplish that target may be used. We used the system of unique personal identification number in both cancer and transplant registers, but in other healthcare systems alternate solutions must be sought to ensure effective MDT evaluations.
In the general population diagnosed with different types of cancer, the recommended oncological treatment was not implemented mainly due to comorbidity in 5–20% of the cases [Citation19]. In patients with colon cancer stage III 19% of the patients with comorbidity were offered chemotherapy compared to 84% of the patients without comorbidity. Still adjuvant therapy resulted in significant prolonged survival even in patients with comorbidity.
In this study we focused on patients with a defined comorbidity, i.e. renal transplantation, and not on patients with a specific cancer type. Overall, the oncological treatment was modified in 52% of the transplanted patients with solid or hematological malignancies. When a change to or addition of mTOR inhibitors as chemotherapeutic agent were added to the adjustments 97% of the patients with solid or hematological malignancies received altered treatment. In non-transplanted populations there is growing evidence that the outcome of patients have improved after implementing MDT decisions [Citation19].
In total 82% of the transplanted patients with solid organ tumors were treated according to national guidelines, with the aid of the MDT strategy, despite the fact that they were transplanted. In the general population the implementation of a planned oncological treatment might be increased from 82% to 92% by training the MDT [Citation23], and this is probably also possible to achieve for transplanted patients.
In conclusion, this is the first study to systematically evaluate patients with a malignancy in combination with a defined comorbidity, i.e. kidney transplantation, at MDT evaluations. We suggest that all transplanted patients with a diagnosed post-transplantation malignancy should be referred to a MDT that addresses and individually tailors the oncological and immunosuppressive treatment. The study indicates that malignant diseases generally are underreported in international transplant registries. We recommend linkage between transplant databases and national tumor registries to improve identification of post-transplant malignancies.
IONC_1130855_Supplemental_material.xlsx
Download MS Excel (41 KB)Acknowledgments
Malin Dackborn, Anne Eriksson, Ingrid Skarp Örberg and Jenny Thulin are acknowledged for their excellent assistance within the project. This study was supported by an unrestricted grant from Novartis AG.
Transparency declarations
The results presented in this paper have not been published previously in whole or part. This study was supported by an unrestricted grant from Novartis AG. No other conflicts of interest exist.
References
- Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 2003;89:1221–7.
- Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant 2010;10:1889–96.
- Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891–901.
- Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008-A Swedish population-based study. Int J Cancer 2013;132:1429–38.
- Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer 2009;125:1747–54.
- Ajithkumar TV, Parkinson CA, Butler A, Hatcher HM. Management of solid tumours in organ-transplant recipients. Lancet Oncol 2007;8:921–32.
- Kauffman HM. Malignancies in organ transplant recipients. J Surg Oncol 2006;94:431–3.
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67.
- Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation 2009;87:1347–59.
- Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation 2010;90:683–7.
- Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant 2007;7:2140–51.
- Kiberd BA, Keough-Ryan T, Clase CM. Screening for prostate, breast and colorectal cancer in renal transplant recipients. Am J Transplant 2003;3:619–25.
- Alberu J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, et al. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011;92:303–10.
- Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 2012;12:1146–56.
- Budde K, Sommerer C, Rath T, Reinke P, Haller H, Witzke O, et al. Renal function to 5 years after late conversion of kidney transplant patients to everolimus: a randomized trial. J Nephrol 2015;28:115–23.
- Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res 2006;66:10040–7.
- Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther 2006;5:1183–9.
- Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ 2010;340:c951.
- Stairmand J, Signal L, Sarfati D, Jackson C, Batten L, Holdaway M, et al. Consideration of comorbidity in treatment decision-making in multidisciplinary cancer team meetings: a systematic review. Ann Oncol 2015;26:1325–32.
- Opelz G, Dohler B. Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation 2009;87:795–802.
- Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation 2006;82:663–8.
- Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer 2004;91:1420–4.
- Lamb BW, Green JS, Benn J, Brown KF, Vincent CA, Sevdalis N. Improving decision making in multidisciplinary tumor boards: prospective longitudinal evaluation of a multicomponent intervention for 1,421 patients. J Am Coll Surg 2013;217:412–20.
- Kidger J, Murdoch J, Donovan JL, Blazeby JM. Clinical decision-making in a multidisciplinary gynaecological cancer team: a qualitative study. BJOG 2009;116:511–17.
- Aapro M, Andre F, Blackwell K, Calvo E, Jahanzeb M, Papazisis K, et al. Adverse event management in patients with advanced cancer receiving oral everolimus: focus on breast cancer. Ann Oncol 2014;25:763–73.
- Opelz G, Dohler B, Ruhenstroth A, Cinca S, Unterrainer C, Stricker L, et al. The collaborative transplant study registry. Transplant Rev (Orlando) 2013;27:43–5.