Abstract
Background HER3 is a member of the human epidermal growth factor receptor complex (EGFR, HER2, HER3 and HER4). It has been investigated as a prognostic biomarker in colorectal cancer but is sparingly studied in colon cancer. HER3 can affect cellular proliferation, differentiation and migration in oncogenesis through ligand binding and activation of intracellular signal pathways. Recently, we found that expression of cell surface HER3 can be detected at a high extent in primary colorectal tumors, lymph node and liver metastases and that it correlated with poor prognosis. This large, explorative, retrospective study evaluates the prognostic value of HER3 in colon cancer and the association of HER3 to tumor location. Material and methods. Immunohistochemical detection with a monoclonal HER3 antibody in primary colon tumors of stage II and III, from 521 patients, was performed. Results HER3 was expressed at high levels in 67% of the colon tumors. High HER3 expression was associated with distal tumor location (p < 0.0001) and low-grade tumor (p < 0.0001). In the group of patients with distal colon cancer (230/521), HER3 expression correlated to shorter disease-free survival (DFS) (p = 0.03) in the univariate analysis and in the multivariate analysis, a hazard ratio of 0.56 (95% CI 0.31–0.99) (p = 0.047) was observed. Conclusion In this explorative, retrospective study, high HER3 expression in colon cancer was associated to distal colon location and low-grade tumor. High HER3 expression was of prognostic value according to DFS in distal colon cancer in univariate and multivariate analysis. We could not find a significant value of HER3 expression with respect to overall survival (OS).
Colon cancer is a common disease affecting a large number of patients worldwide [Citation1]. The most frequently used staging system today is the continuously updated Tumor/Node/Metastases (TNM) system from the American Joint Committee on Cancer (AJCC) [Citation1]. Clinical pathological staging of colon cancer remains the standard for assessing patient risk and supporting treatment decisions. There is a need for improvement in identifying biological and molecular characteristics of the disease; accurate prognostic and predictive markers that could enhance survival and sharpen cancer therapy.
Growth factors and their receptors take part in regulation of cellular proliferation, differentiation and migration during both embryogenesis and oncogenesis. The human epidermal growth factor receptor (EGFR/HER) complex consists of EGFR (HER1, ErbB1), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4) [Citation2]. The receptors are embedded in the plasma membrane of the cell and are composed of an extracellular ligand binding domain, a transmembranous domain, and an intracellular domain with tyrosine kinase catalytic ability. After ligand binding, the receptors dimerize and their effect is exerted by activation of intracellular signaling pathways. There are several pathways that function through the HER complex, e.g. MAPK pathway, PI3K pathway and the STAT pathways. Over expression of EGFR, HER2 and HER3 has been linked to oncogenic transformation [Citation2].
The HER2 receptor did turn out to be clinically relevant in breast cancer when over expressing HER2 through gene amplification was associated with a more aggressive tumor biology and poor prognosis [Citation3]. While EGFR, HER3 and HER4 are activated by extracellular ligands, HER2 is an orphan receptor and is non-stop active [Citation4]. The HER2/HER3 heterodimer have particularly high signaling capacity [Citation5].
Neuregulins are extracellular ligands of the HER3 receptor. HER3 is tyrosine kinase domain deficient and cannot be active as a homodimer but can form potent heterodimers. Inappropriate signaling can be an effect of receptor over expression. Signaling can also be affected by interdependency between different members of the HER complex [Citation6]. High expression of HER3 is an established negative prognostic biomarker in some malignancies, e.g. breast, gastric and ovarian cancer [Citation7]. Cancer therapies for solid tumors in which monoclonal antibodies are used, e.g. cetuximab (anti-EGFR) or trastuzumab (anti-HER2), to target the HER complex are becoming important parts of the oncological treatment. HER3 inhibiting antibodies are under development and already in early clinical trials [Citation8]. Regarding the significance of HER3 expression in colorectal cancer (CRC), the results are few and differ in prognosticity [Citation7,Citation9]. HER3 expression in exclusively colon cancer is even less studied.
The primary aim of this explorative, retrospective study was to evaluate HER3 expression in a large patient material of primary colon cancer of stage II and III and hereby investigate the reliability of HER3 as a prognostic marker. The secondary aim was to examine the distribution of high HER3 expression regarding proximal and distal tumors and identify prognostic diverging groups other than stage II and III.
Material and methods
Patients
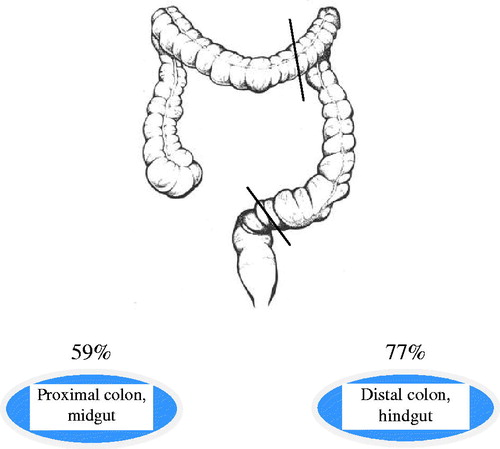
The primary tumors of 521 patients, with radically resected colon cancer of stages II and III from 29 hospitals in Sweden, were analyzed with respect to HER3 expression. The surgery was performed between 1991 and 1997 and the minimum follow-up time was five years. The last patient update was in November 2004. Surgical specimens were derived from adjuvant Nordic trials, where patients (≤75 years) with CRC of stage II and III were randomized to either surgery or surgery plus 5 fluorouracil (5-FU) based adjuvant chemotherapy [Citation10]. The 521 patients were a part of the Swedish cohort of colon cancer patients in the Nordic trials. The patient material in this study included 172 colon cancer cases from our pilot study and was then enlarged up to 521 patients [Citation9]. The Dukes staging system and the stage I–IV system for colon cancer were used in the pathology reports. The definition of proximal colon location was tumors in the cecum, ascending and transverse colon. The definition of distal colon location was left flexure, descending and sigmoid colon. Regarding tumor differentiation, low-grade tumor represents a well to moderately differentiated tumor while a high-grade tumor represents poor differentiation. The definition of disease-free survival (DFS) was time from surgery to recurrence or to death of any cause. Clinical data were retrieved from regional centers of epidemiological oncology in Sweden. The patient demographics and tumor characteristics are displayed in . This study conforms to guidelines for prognostic tumor marker research, the REMARK criteria [Citation11] and was approved by the local Ethical Committee at the Karolinska Institutet.
Table 1. Patients and tumor characteristics.
Immunohistochemical analysis
The examined colon specimens were derived from formalin-fixated, paraffin-embedded (FFPE) tumors in 4-μm slices. Sections were deparaffinized in xylene, rehydrated in ethanol and washed in distilled water. For antigen retrieval, slides were boiled in Dakos Retrievel, pH 9.0, in a pressure cooker (2100 Retriever) and rinsed twice. Endogenous peroxidation in the tumor section was quenched by treatment with 3% hydroperoxidase. Background staining was reduced by incubation in 10% goat serum for 30 minutes. A HER3 monoclonal antibody from rabbit (Abcam, SP71 ErbB, ab 93739, dil 1:800) was added and left over night at +4°C. Samples were rinsed and incubated with an amplification system, EnVision™, HRP system (DakoCytomation) for 30 minutes. Visualization of staining with 3,3-diaminobenzidine tetrahydrochloride (DAB, DakoCytomation) was carried out followed by Mayers Hematoxylin staining.
Evaluation of immunohistochemistry
The HER3 receptor was mainly expressed in the cell membrane and only a small fraction was present in the cytoplasm [Citation6]. The grading was therefore done of the membrane stain. The intensity of the membrane stain in cancer cells was graded 0–3. Absent stain or occurrence of staining of any grade in <10% was categorized as grade 0. Very faint membrane staining, present in only part of the membrane, was considered grade 1. Weak to moderate stain that was complete (circumferential) in the cell membrane or basolateral, was grade 2 and strong stain, complete or basolateral was defined as grade 3 (). Cytoplasmic or background staining was not graded.
Figure 1. HER3 expression, IHC, grade 0 and grade 3 detected by HER3 monoclonal rabbit antibody (Abcam, SP71 ErbB, ab 93739, dil 1:800).
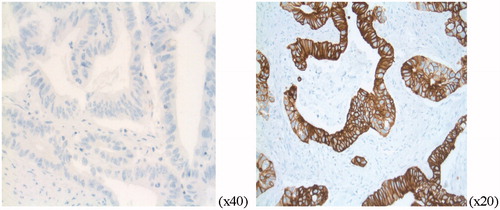
Grades 0–1 were categorized as low expression and grades 2–3, as high expression of membranous HER3. Slides from the primary colon tumor were evaluated using a light microscope by two independent observers (F.L and M.H). Disagreements (<5%) were reviewed followed by conclusive judgment. The entire FFPE section of the tumor was evaluated and an approximation was done on the percentage of stained cancer cells. Staining pattern was scored according to the Hercept test, DAKO interpretation manual for gastric cancer since no established scoring of HER3 in colon cancer exists.
Statistics
Differences in distribution between groups were compared using the Chi2-test which also used to examine the relationships between patient’s demographics, tumor characteristics and HER3 expression. Survival analysis was performed using the Kaplan-Meier method. Correlation analysis was done with the Spearman’s ranks test. Cox regression was used in the univariate and multivariate analysis. The results were considered significant if p < 0.05 and tests were two-tailed. Calculations were performed using Statistica version 10 (StatSoft, Tulsa, OK, USA).
Results
Patient and tumor characteristics
This study included 521 colon cancer patients with stage II and III disease. The median age was 66 years. The follow-up time was up to 120 months and median survival time was 82 months (range 2–120 months). The patients and tumor characteristics are shown in .
HER3 expression and clinicopathological parameters
Sixty-seven percent of the tumors showed high expression of HER3 (grade 2–3). The HER3 expression in grades 0–3 can be seen in . There was no significant difference in HER3 expression between stage II and III. HER3 expression was independent of sex and age.
Table 2. HER3 expression grades in colon cancer, high vs. low expression.
HER3 expression related to location of tumor
High HER3 expression was associated with tumor location (p < 0.0001) (). In proximal colon tumors, high HER3 expression was seen in 171/291 (59%) compared to 176/230 (77%) in distal tumors (). The association between high HER3 and distal location was seen in both stage II (p < 0.0001) and stage III (p = 0.01).
HER3 expression according to tumor grade
There was a correlation between high HER3 expression and low-grade tumor (p < 0.0001) (). In the group of low-grade tumors, 280/394 (71%) expressed high levels of HER3 compared to 51/104 (49%) in the group of high-grade tumors. The association between high HER3 expression and low-grade tumor was significant in the whole group of patients and in stage III (p < 0.0001) but not for stage II.
HER3 expression and combined tumor location and grade
A higher amount of distal colon tumors were categorized as low-grade cancers 195/222 (88%) compared with proximal tumors 199/276 (72%), (p < 0.0001). In the group of 394 patients with low-grade tumor, there were a larger proportion of distal tumors with high HER3 expression, 153/195 (78%), compared with proximal tumors, 127/199 (64%). In the multivariate analysis, we found that a higher frequency of distal colon tumors have high HER3 expression compared with proximal, independent of tumor grade, OR = 2.09 (95% CI 1.39–3.14), (p = 0.001).
HER3 expression and clinical outcome
HER3 expression in the primary colon tumor was not found to be prognostic for overall survival (OS), neither in the entire group, nor in subgroups of different stage, treatment, localization or tumor grade. High HER3 expression was associated with shorter DFS in patients with distally located colon tumors (n = 176/230) [HR = 1.73 (95% CI 1.06–2.81), p = 0.03] (). In the multivariate analysis of HER3 expression and DFS in distal tumors, a hazard ratio of 0.56 (95% CI 0.31–0.99) and p = 0.047 was observed (). Multivariate analysis showed that stage, age and number of analyzed lymph nodes ≥12 correlated independently to OS in the entire group of patients. We also found in the univariate model that stage (p = 0.002) and sex (p = 0.04) correlated to DFS in distal colon tumors but only sex and HER3 expression remained significant in the multivariate analysis ().
Figure 3. Disease-free survival in proximal and distal tumor location with respect to HER3 expression. For distal colon, the difference in DFS between low and high HER3 expressing tumor is significant (p = 0.03).
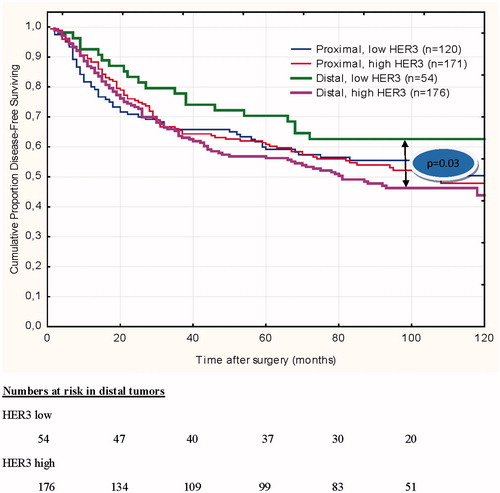
Table 3. Multivariate analysis for disease-free survival in distal colon tumors (n = 230).
Discussion
In this explorative, retrospective study, we have found that two thirds of the colon cancer tumors showed high membranous HER3 expression detected by immunohistochemistry. The HER3 expression was of prognostic value with respect to DFS in patients with distal colon cancer where high expression correlated to a worse outcome. High HER3 expression was associated independently to distal colon location and to low-grade tumor.
In general, IHC is subjectively quantitative through a narrow range but widely used in the clinic and reliable when validated by other protein analyzing methods, e.g. mass spectrometry or western blotting. The proteins in the HER complex, including HER3, are so far lacking robust and validated assays for detection [Citation12]. IHC quality is depending on various factors like specimen fixation time, different staining protocols, quality and specificity of antibodies and scoring methods.
This is a comparatively large explorative, retrospective study on HER3 expression in colon cancer. The tumor material is from a randomized, adjuvant trial and is homogenous and includes only radical resected, colon cancer patients. Some studies tend to mix primary and metastatic disease, colon and rectal cancer, pre- and post-operatively given chemotherapy. As a result of representative tumor material, reviewed by an affiliated pathologist and a well working antibody SP71 (ErbB, ab 93739), the staining resulted in very few IHC failures. The 521 patients were not expected to differ from the colon cancer cohorts in other Nordic countries regarding age, gender and treatment [Citation10]. However, an overweight of stage III patients were known in our material compared to the Nordic trials. A weakness in our study is that tumors were collected from several hospitals and specimen routines might differ before paraffin embedding. The patients underwent surgery in the 1990s when fewer lymph nodes were counted and this can result in under staging. Another weakness is that the original histopathology reports were used, but as mentioned a validation by a pathologist has been performed.
Previously, we reported a study of 236 CRC patients (172 colon and 64 rectum) where high HER3 expression was prognostic regarding OS in the entire group of patients and in the subgroup of stage II colon cancer [Citation9]. These findings led to this unique, enlarged and refined study of colon cancer, stage II and III. We included only colon cancer to avoid unexpected events of HER3 expression caused by radiation therapy. HER3 expression did not prove to be prognostic with respect to OS for the entire group of patients, but prognostic for DFS in the group of distal colon cancers in uni- and multivariate analyzes. When comparing DFS with another parameter, cancer-related death, no difference was seen.
So why is HER3 expression in distal colon tumors prognostic regarding DFS but not for OS? DFS is an approved way of monitoring prognosis in colon cancer [Citation13]. Plausible affecting factors are a low number of patients in the different subgroups and that the prognostic power of HER3 is not so strong. The finding that high HER3 expression is more prevalent distally and decreases in high-grade tumors might have some connection with, e.g. microsatellite instability (MSI) status that presents the inverse relationship (proximal and high-grade tumors). A meta-analysis by Ocana et al. investigated HER3 expression, assessed by IHC (including both membranous and cytoplasmic staining) in solid tumors related to survival. Three CRC studies were included. Two of the studies showed that high HER3 was not prognostic for OS and one study showed that high HER3 was prognostic for shortened OS. The final conclusion for solid tumors was that HER3 was a prognostic factor [Citation7]. Ljuslinder et al. examined HER3 expression using IHC in a patient material of CRC (n = 48) and found about the same percentage of tumors presenting high HER3 expression as in our previous reported study and in this study [Citation14]. In another report (included in the above mentioned meta-analysis), Kountourakis et al. found HER3 membrane expression in only 18/106 (17%) of the tumors examined. High HER3 expression was associated to moderate differentiation of the tumor, similar to our finding [Citation15]. Both Ljuslinder and Kountourakis included not only colon cancer but CRC and the numbers of patients were low.
We report high HER3 expression in 67% of colon tumors. In our previous study, 63% of the colon tumors had high HER3 expression and, notably, the rectal tumors showed high HER3 expression in 87.5% of the tumors [Citation9]. The association between tumor localization in the colon and HER3 expression is a novel finding in colon cancer (). We additionally found that a higher number of distal colon tumors have high HER3 expression not only due to that distal tumors are more frequently low-grade tumors. This is in analogy with findings by Missiaglia et al. where expression of EGFR and HER2 correlated with distal tumor location [Citation16]. Also K-Ras mutation has been linked to colon tumor location [Citation16,Citation17]. There are differences between proximal, distal colon and rectal tumors that may have an impact on prognosis. The distinction between the colon and rectum is to a large extent anatomical, but surgical and radiotherapeutic management differs. The embryological definition of the border between midgut and hindgut is at two thirds of the transverse colon. Tumor location is a source of biological heterogeneity potentially with prognostic and predictive implications [Citation18–20].
A common gross classification of colon cancer subtypes are tumors with microsatellite instability and the epigenetic, hypermethylated phenotype (∼15%, MSI/CIMP) and those that are microsatellite stable, but chromosomally unstable (∼85%, CIN). It is important to tie together anatomical, histological and molecular interplay and connect it to novel targets.
Has target ability of HER3 been under estimated? In this report, and in our previous studies, we have shown that a majority of the colon and rectal tumors and its metastases over express HER3 at the cell membrane. There is increasing evidence that HER3 expression in colon cancer can be of importance as a predictive biomarker for biological therapy. Cushman and Gaborit report a predictive value of HER3 expression in CRC patients treated with targeting monoclonal antibodies. When regarding upcoming anti-HER3 agents, this area needs to be investigated further [Citation21,Citation22]. Selecting therapy in a more stratified, individual approach has been successful in breast cancer HER2 animal models by defining molecular subtypes that respond differently to treatments. This might be applicable for CRC, also a solid tumor dependent on the HER axis [Citation23]. Nakata et al. suggests that HER3 deficiency, EGFR/HER2 proficiency and poor differentiation enhance the gefitinib effect in CRC cells [Citation24]. Gala et al. shows that when resistance to chemotherapy (5-FU + irinotecan) occurs, a patient with HER3 expression in breast cancer can benefit from cetuximab treatment [Citation25].
No predictive value of HER3 was observed in the patients receiving chemotherapy, but our study was not designed to answer this question. In the adjuvant study from which our subgroup of patients originated, the survival benefit of chemotherapy did not reach statistical significance [Citation10].
CRC is probably composed of a spectrum of different molecular subtypes. The detection of HER complex and HER3 can be of importance. Large, randomized studies and innovative trial design will be needed to provide the evidence base that will enable clinicians to determine optimal combinations of treatments of colon respectively rectal cancer patients.
In conclusion, we found in this explorative, retrospective study that distal location and low-grade colon tumor did associate with HER3 expression. HER3 was a prognostic biomarker in distal colon cancer for DFS in uni- and multivariate analyzes, but not for OS. It remains to explore if HER3 correlates with other biomarkers in colon and rectal cancer and if there is predictive potential of HER3 while giving antibody treatments like, e.g. cetuximab.
Acknowledgments
We would like to thank Hemming Johansson for contributing to the statistical analysis. Thanks also to the colleagues at the Clinical Pathology Division at Karolinska University Hospital, Associate professor Göran Elmberger and MD, PhD, Anna Kwiecinska. This work was supported by grants from The Ihre Foundation of the Swedish Society of Medicine, The Juhlin Foundation, Syskonen Svenssons Foundation and The Cancer Research Foundations of Radiumhemmet and regional agreement on medical training and clinical research (ALF) between Stockholm county council and the Karolinska Institutet.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- National Cancer Institute, www.cancer.gov/types/colorectal/hp/colon-treatment-pdq. 2015.
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: A model for targeted therapy. Clin Cancer Res 2006;12:5268–72.
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, Mcguire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82.
- Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 2000;19:6102–14.
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. Embo J 1997;16:1647–55.
- Beji A, Horst D, Engel J, Kirchner T, Ullrich A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin Cancer Res 2012;18:956–68.
- Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013;105:266–73.
- Huang J, Wang S, Lyu H, Cai B, Yang X, Wang J, et al. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer 2013;12:134.
- Lédel F, Hallström M, Ragnhammar P, Ohrling K, Edler D. HER3 expression in patients with primary colorectal cancer and corresponding lymph node metastases related to clinical outcome. Eur J Cancer 2013;50:656–662.
- Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, et al. Adjuvant chemotherapy in colorectal cancer: a joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol 2005;44:904–12. Erratum in: Acta Oncol. 2006;45(1):110.
- Mcshane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics, et al. Reporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol 2005;2:416–22.
- Nuciforo P, Radosevic-Robin N, Ng T, Scaltriti M. Quantification of HER family receptors in breast cancer. Breast Cancer Res 2015;17:53.
- Sargent D, Wieand H, Haller D, Gray R, Benedetti J, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23:8664–70.
- Ljuslinder I, Malmer B, Isaksson-Mettavainio M, Oberg A, Henriksson R, Stenling R, et al. ErbB 1-4 expression alterations in primary colorectal cancers and their corresponding metastases. Anticancer Res 2009;29:1489–94.
- Kountourakis P, Pavlakis K, Psyrri Rontogianni AA, Xiros DN, Patsouris E, et al. Prognostic significance of HER3 and HER4 protein expression in colorectal adenocarcinomas. BMC Cancer 2006;6:46.
- Missiaglia E, Jacobs B, D'ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995–2001.
- Greystoke A, Mullamitha SA. How many diseases are colorectal cancer? Gastroenterol Res Pract. 2012;2012:564741.
- Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - A systematic review. Eur J Surg Oncol 2015;41:300–8.
- Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, Colon/Rectum Carcinomas (Primary Tumor) Study Group. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010;53:57–64.
- Brule SY. Jonker DJ, Karapetis CS, O’Callaghan CJ, Moore MJ, Wong R, et al. Location of colon cancer (right-sided [RC] versus left-sided [LC]) as a predictor of benefit from cetuximab (CET). J Clin Oncol 31, (suppl; abstr 3528). Epub 2013 ASCO Annual Meeting.
- Cushman S, Jiang C, Hatch A, Shterev I, Sibley A, Niedzwiecki D, et al. Gene expression markers of efficacy and resistance to cetuximab treatment in metastatic colorectal cancer: results from CALGB 80203 (Alliance). Clin Cancer Res 2015;21:1078–1086.
- Gaborit N, Abdul-Hai A, Mancini M, Lindzen M, Lavi S, Leitner O, et al. Examination of HER3 targeting in cancer using monoclonal antibodies. Proc Natl Acad Sci USA 2015;112:839–44.
- Ma J, Lyu H, Huang J, Liu B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 2014;13:105 Review.
- Nakata S, Tanaka H, Ito Y, Hara M, Fujita M, Kondo E, et al. Deficient HER3 expression in poorly-differentiated colorectal cancer cells enhances gefitinib sensitivity. Int J Oncol 2014;45:1583–93.
- Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res 2014;20:1410–6.