Abstract
Purpose The time course of changes of the liver function after stereotactic body radiotherapy (SBRT) was analyzed in patients treated for non-resectable hepatocellular carcinoma (HCC).
Patients and methods Twenty-six patients with non-resectable HCC treated with SBRT were included in this study. Clinical, biochemical and treatment-related parameters were retrospectively collected. S-albumin, s-bilirubin, s-alkaline phosphatase (AP) and s-alanine transaminase (ALAT) at 0, 3, 6, and 12 months after radiotherapy were analyzed.
Results Seventeen and nine patients were Child-Pugh class A and B, respectively. The liver was exposed to relatively high radiation doses with mean doses of 1.9–26 Gy. None of the patients developed classic radiotherapy-induced liver disease (RILD), but two patients developed non-classic RILD. Two patients developed grade 3 ascites and no grade 4-5 toxicities were observed. Six patients declined in Child-Pugh class. The s-albumin decreased significantly from a pretreatment median of 37.4–34.36 g/l at three months after SBRT and stabilized thereafter. S-bilirubin, s-AP and s-ALAT did not change significantly over the study period.
Conclusion Despite the fact that patients received high radiation dose to the liver, there was only moderate morbidity related to the treatment. The s-albumin decreases over three months after SBRT reflecting minor to moderate hepatic toxicity. S-albumin should be observed in the follow-up of HCC patients treated with SBRT.
Hepatocellular carcinoma (HCC) is a challenging cancer. Globally, it is the third leading cause of cancer death [Citation1]. Surgical resection or liver transplantation are standard treatment options for early stage HCC, but the large proportion of patients are ineligible for surgery. With the technological advances, radiotherapy (RT) is an important option in the treatment of selected HCC patients [Citation2]. By stereotactic body radiation therapy (SBRT) it is possible to deliver a high radiation dose to the tumor, while at the same time to preserve sufficient liver function. Several studies show that SBRT can be used safely as a therapeutic option for patients with primary and secondary liver tumors including HCC [Citation2–4].
The liver is believed to have a relatively low radiation tolerance and this is a major limitation for the use of SBRT in therapy of HCC [Citation5]. Most patients with primary liver cancer and especially HCC have hepatitis B, C or alcoholic cirrhosis with subsequent impaired liver function, and they are considered to have an even lower tolerance of radiation to the liver. Radiation to the liver may result in radiotherapy-induced liver disease (RILD) [Citation6–8]. RILD is a serious condition and death due to radiation therapy has been reported in the literature after conventional RT as well as after SBRT [Citation7,Citation8]. It is also a very challenging condition, as there are few or no clinical characteristics related to its early phase; once the clinical signs appear, it is most often too late to intervene.
The present study aims to elucidate on the relationship between biochemical changes in the blood reflecting the liver function after SBRT of patients treated for HCC with Child-Pugh A and B liver cirrhosis.
Patients and methods
Patient characteristics
Twenty-six patients with non-resectable HCC, treated with SBRT at Aarhus University Hospital between October 1999 and October 2013 were included in the analysis. Only patients with available blood samples were included. The clinical data was retrospectively retrieved from the patient charts and laboratory data from the clinical laboratory information system.
HCC was diagnosed by histological examination in seven patients. In 19 patients the diagnosis was based on European Society for Medical Oncology (ESMO) guidelines of diagnosis and staging, including radiological modalities and s-alpha-fetoprotein (AFP) values [Citation9]. The multidisciplinary liver tumor board at Aarhus University Hospital reviewed all patients before reference to SBRT.
Stereotactic body radiation therapy
Patients were immobilized by use of a stereotactic body frame with an abdominal compression device (SBF, Elekta, Stockholm, Sweden). Treatment planning was carried out by use of the Helax-TMS (MDS-Nordion, Uppsala, Sweden) treatment planning system (TPS) until 2006 (n = 17) and from 2007 on the Eclipse TPS (Varian Oncology, Palo Alto, CA, USA) was used (n = 9). The gross tumor volume (GTV) was defined by the visible tumor on an arterial phase contrast enhanced CT-scan. In four cases, MRI was also available. The clinical target volume (CTV) was equal to the GTV. The planning target volume (PTV) was defined by addition of 5 mm in the horizontal plane and 10 mm in the cranio-caudal plane. In case of large respiratory motion, the margins were increased accordingly. Thus, in some patients the PTV was defined by expansion of the CTV by up to 20 mm. Typically, 5–7 coplanar and non-coplanar beams were used. Nineteen patients received 3 × 15 Gy = 45 Gy, three patients received 3 × 18.75 Gy = 56.75 Gy, one patient 6 × 10 Gy = 60 Gy, one patient 3 × 22.5 Gy = 67.5 Gy, one patient 2 × 15 Gy = 30 Gy and one patient 1 × 18.75 Gy = 18.75 Gy. The two patients receiving 1–2 fractions were scheduled to receive 3 fractions, but the treatment was interrupted due to gastric bleeding or deterioration. The radiation dose was prescribed as the Dmean to the CTV allowing a dose between 95% and 107% to the CTV and the PTV was covered by the 67% iso-dose line. At least 700 cm3 of the liver should receive a radiation dose below 15 Gy and the mean liver dose should be kept as low as possible. D4cm3 (maximum dose to 4 cm³ of the volume) of tubular structures was lower than 27 Gy. Treatment was given on a Siemens Primus (Siemens Medical Solutions, Concord, CA, USA) or on a Varian Trilogy (Varian Oncology) linear accelerator, respectively.
Evaluation
Blood tests were analyzed in all 26 patients and dose-volume histogram (DVH) parameters in 19 patients. Results of blood tests [s-albumin, s-bilirubin, s-alkaline phosphatase (AP) and s-alanine transaminase (ALAT) before RT starts and 3, 6 and 12 months after RT] were retrieved from the patient charts and the electronic laboratory report system. Morbidity was evaluated by the CTCAE version 4.0 grading system and the severity of the cirrhosis by the Child-Pugh classification [Citation10–12]. The principal volumes and dose parameters related to GTV, PTV, and the liver, as well as the mean dose in the liver was retrieved from the hard copy RT report (Helax planned cases) and from the TPS (Eclipse planned cases).
Statistical analysis
The Wilcoxon signed rank test was used for differences between groups. Significance was defined by a level of the p-value below 0.05 and all tests were two-sided. The Kaplan-Meyer curve was used to demonstrate overall survival after the first fraction of SBRT. The SPSS software package (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Of the total of 26 patients, 17 patients were classified as Child-Pugh A 5-6 and nine patients as Child-Pugh B 7-8 before SBRT. Median s-AFP level was 11 kIU/l (range 2–65 703 kIU/l). Median age of the patient group was 64.5 years (range 40–85 years). Patient characteristics are given in . DVH-parameters are listed in . Dmean to the liver was 9.0 Gy in median. Dmean had a range of 1.9–26 Gy.
Table 1. Patient characteristics.
Table 2. Summary of DVH-parameters.
Five patients developed encephalopathy grade 1, one patient developed hepatomegaly grade 1, and three patients developed ascites, two grade 3, one grade 1 according to the CTCAE grading system [Citation10]. For the patient with grade 1 ascites no DVH-parameters are available. For the two patients with grade 3 ascites Dmean to the liver was 24.9 Gy and 5.7 Gy, Dmean to the liver minus target volume was 23.0 Gy and 5.1 Gy.
Six patients declined in Child-Pugh score: one patient from 5-A to 6-A, one patient from 5-A to 7-B, two patients from 6-A to 7-B, one patient from 6-A to 8-B, and one patient from 6-A to 10-C. All patients had a decrease in s-albumin after SBRT. The patient with Child-Pugh score C after SBRT presented with an increased international normalized ratio (INR) of 2.2, hypoalbuminemia of 30.6 g/l, hyperbilirubinemia of 59 μmol/l, ascites grade 3, but signs of encephalopathy did not occur (grade 0). This patient received 3 × 18.75 = 56.75 Gy. Dmean to the liver and liver minus CTV were 24.9 Gy and 22.9 Gy, respectively. Due to worsening of Child-Pugh score by ≥2 (in the absence of classic RILD), this patient fulfilled the criteria of non-classic RILD (). The patient with a decline in Child-Pugh score from 6-A to 8-B fulfilled the criteria of non-classic RILD as well. None of the patients developed classic RILD.
Table 3. RILD.
For all patients s-bilirubin, s-AP, and s-ALAT did not increase significantly after SBRT (). The red lines indicate the normal range of the blood parameters. With regard to the CTCAE v4.0 criteria for treatment-related changes in blood parameters [Citation10], one patient developed grade 2 increased s-bilirubin, one grade 3, and one grade 4, respectively. One patient developed grade 1 increased s-ALAT, and one grade 2, respectively. Two patients developed grade 1 increased s-AP, three patients grade 2, and two patients grade 3.
Figure 1. S-albumin (A), s-bilirubin (B), s-alkaline phosphatase (C) and s-alanine transaminase (D) over time for 26 patients treated with SBRT for HCC.
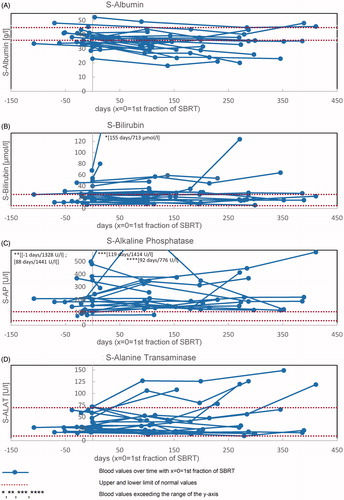
The median s-albumin level before RT was 37.4 g/l (range 23.0–52.3 g/l; SD = 6.1 g/l), measured with a median of 12 days in advance of the first SBRT fraction. After a median of 101 days after the first SBRT fraction, the median s-albumin level decreased significantly 8% to 34.4 g/l (range 18.0–49.2 g/l; SD = 7.0 g/l) (p = 0.003). The time course of s-albumin changes is shown in . The red lines indicate the normal range of s-albumin from 36 to 45 g/l. With regard to the CTCAE v4.0 criteria [Citation10]: Three patients developed grade 1 hypoalbuminemia, six patients developed grade 2, and one patient developed grade 3, respectively. On top of that, the patients can be divided into two subgroups: 15 patients with s-albumin values above lower limit of normal, and 11 patients with s-albumin values under lower limit of normal before SBRT. From the first subgroup three patients developed grade 1 and one patient grade 2 hypoalbuminemia. From the second subgroup five patients developed grade 2 and one patient grade 3 hypoalbuminemia.
S-albumin did not change significantly from 3 to 6 or 6 to 12 months after SBRT, but it did change significantly with regard to pretreatment (p = 0.005 and p = 0.028), that is to say the s-albumin level decreased from base-line to three months post-SBRT and stabilized thereafter.
The Kaplan-Meyer curve in shows overall survival after the first fraction of SBRT. Three months after the first fraction of SBRT 23 patients, six months after the first fraction of SBRT 20 patients and 12 months after the first fraction of SBRT 17 patients were still alive. One patient was still alive at the end of our analysis (32 months after first fraction of SBRT). None of our patients became lost to follow-up.
Discussion
RILD is an important factor that limits the utilization of SBRT especially in therapy of liver cancer [Citation7,Citation13]. RILD – both classic and non-classic – is a severe condition for patients being treated by RT. Radiation to the liver induces a hepatic veno-occlusive disease [Citation14–17] (however, most often sparing the larger veins), and activation of hepatic stellate cells causes atrophy, loss of hepatocytes and formation of fibrosis. This is a life threatening condition, which can progress to liver failure. Treatment is mainly supportive and consists of diuretics and steroids. The majority of patients will recover with supportive treatment, but RILD may lead to death. Therefore, an early identification of threatening RILD is of great importance.
The fear of RILD is the reason for a considerable underuse of SBRT in the therapy for primary and secondary liver cancer. However, the liver has a pronounced volume effect and small volumes may be irradiated to very high doses without compromising the organ function [Citation6]. Hepatitis carriers and cirrhosis patients have lower radiation tolerance to the liver [Citation6] and deaths from RILD have been reported in the literature [Citation15]. For this reason, Herfarth et al. proposed constraints of V12 ≤ 30% and V7 ≤ 50%, and the Quantitative Analyzes of Normal Tissue Effects in the Clinic (QUANTEC) paper by Pan et al. recommended that Dmean to the liver minus CTV should be kept below 13 Gy in the treatment of primary liver cancer [Citation5,Citation18].
Patients treated early on in the present study were allowed to receive considerably higher median liver doses and it is important to notice that SBRT of these patients were carried out without observation of serious adverse effects. However, due to the retrospective nature of the present study and the lack of 3D dose-volume data, we are only able to demonstrate the morbidity in relation to the Dmean to the liver without exclusion of the CTV.
None of our patients experienced classic, two patients experienced non-classic RILD as a complication after SBRT. Nevertheless, we were able to show a small, but significant decrease in metabolic function in terms of reduced s-albumin levels and a decline in Child-Pugh score after SBRT in some of the patients. The decline in Child-Pugh score exceeds the results from Dyk et al., concluding that about one quarter of patients after SBRT for liver malignancies experience a worsening in Child-Pugh score [Citation19]. Bilirubin, ALAT, and AP did not change systematically over time. Albumin level decreased significantly during the first three months after SBRT and stabilized thereafter at a lower level. The patients with decline in Child-Pugh score had a decreased s-albumin level after SBRT. This can be explained by a radiation-induced reduction of liver function, that does not seem to recover due to the lack of regeneration capability in cirrhotic liver.
There is only limited knowledge on the development of RILD and on the radiation tolerance of the liver. The symptoms and biochemical tests to foresee the development of RILD are not clearly defined. Due to the severity of the condition, physicians tend to be conservative while determining radiation strategies for patients with liver cancer. This study indicates that SBRT with relatively high radiation dose to the liver is feasible in Child-Pugh A and B patients if they are well selected. However, the treatment planning should aim at the lowest possible dose to the liver and the patients followed carefully after the treatment.
Due to the sparing of the low and intermediate dose wash, particle therapy may be ideal for treatment of HCC [Citation20]. We need to establish methods for selection of the proper patients for particle therapy. Treatment planning studies using normal tissue complication probability modeling (NTCP) is a way to compare protons and photons [Citation21]. However, the relevant input parameters to the NTCP model have so far not been established. NTCP modeling may have great potential in risk adapted dose prescription of SBRT of liver tumors. It may be employed in comparative treatment planning studies between particle- and photon-based SBRT for optimal selection of candidates for particle beam therapy, that may offer ideal dose distribution and favorable relative biological efficacy [Citation20,Citation22–24].
Conclusion
The present study showed that SBRT with high radiation dose resulted in minor change in liver function and seems feasible in treatment of patients with Child-Pugh class A and some patients with class B. S-albumin was the only blood test that changed systematically during a three-month period and it stabilized thereafter. The findings indicate that the decrease in s-albumin reflects a minor RILD.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90
- Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–9.
- Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657–64.
- Habermehl D, Herfarth KK, Bermejo JL, Hof H, Rieken S, Kuhn S, et al. Single-dose radiosurgical treatment for hepatic metastases - therapeutic outcome of 138 treated lesions from a single institution. Radiat Oncol 2013;8:175.
- Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol 2010;76:S94–100
- Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 2005;15:279–83.
- Jung J, Yoon SM, Kim SY, Cho B, Park JH, Kim SS, et al. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 2013;8:249.
- Cheng JC, Wu JK, Huang CM, Liu HS, Huang DY, Cheng SH, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol 2002;54:156–62.
- Jelic S, Sotiropoulos GC, Group Obot EGW. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v59–64.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS. May 29, 2009. NIH publication # 09-7473.
- Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastro 1989;24:269–76.
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Problems Clin Surg 1964;1:1–85
- Cheng JC, Wu JK, Huang CM, Huang DY, Cheng SH, Lin YM, et al. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol 2002;63:41–5
- Olsen CC, Welsh J, Kavanagh BD, Franklin W, McCarter M, Cardenes HR, et al. Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol2009;73:1414–24.
- Sempoux C, Horsmans Y, Geubel A, Fraikin J, Van Beers BE, Gigot JF, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology 1997;26:128–34.
- Jeffrey RB, Jr., Moss AA, Quivey JM, Federle MP, Wara WM. CT of radiation-induced hepatic injury. Am J Roentgenol 1980;135:445–8.
- da Silveira EB, Jeffers L, Schiff ER. Diagnostic laparoscopy in radiation-induced liver disease. Gastrointestin Endosc 2002;55:432–4.
- Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001;19:164–70.
- Dyk P, Weiner A, Badiyan S, Myerson R, Parikh P, Olsen J. Effect of high-dose stereotactic body radiation therapy on liver function in the treatment of primary and metastatic liver malignancies using the Child-Pugh score classification system. Pract Radiat Oncol 2015;5:176–82.
- Habermehl D, Debus J, Ganten T, Ganten MK, Bauer J, Brecht IC, et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma - feasibility and clinical response. Radiat Oncol 2013;8:59.
- van de Water TA, Lomax AJ, Bijl HP, Schilstra C, Hug EB, Langendijk JA. Using a reduced spot size for intensity-modulated proton therapy potentially improves salivary gland-sparing in oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2012;82:e313–9.
- Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol 2006;45:856–64.
- Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011;117:4890–904.
- Petersen JB, Lassen Y, Hansen AT, Muren LP, Grau C, Hoyer M. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol 2011;50:823–8.

![Figure 2. Kaplan–Meyer curve of overall survival [in months] of 26 patients after the first fraction of SBRT for HCC.](/cms/asset/5c2e3fb3-29f4-49a2-b5e4-e9b928ebbfb5/ionc_a_1137352_f0002_c.jpg)