To the Editor,
Treating head and neck cancer (HNC) is challenging as the tumors are located in a critical area where many essential functions originate. Amongst the late appearing side effects after radiotherapy (RT) for HNC, restricted mouth opening (trismus) occurs in more than one third of patients and have been described as persisting or even worsening five years after completed RT [Citation1–3]. Trismus can lead to difficulties in eating, chewing, maintaining oral hygiene, and potentially to malnutrition and weight loss [Citation2–6].
Irradiation of the temporomandibular joint (TM joint) and muscles of mastication, especially doses to the masseter and the pterygoid muscles, have been reported to be associated with radiation-induced trismus [Citation7–11]. However, available data diverge as to which anatomical structure is more critical for trismus after RT in HNC. The aim of this prospective study was, therefore, to investigate if the use of both objectively and subjectively determined trismus can shed further light on this issue.
Material and methods
Additional information on the study population (inclusion/exclusion criteria), treatment, and statistics can be found in the appendix (available online at http://www.informahealthcare.com).
Study population
This study includes patients treated for HNC with external beam RT (EBRT) only or EBRT in combination with brachytherapy, with or without chemotherapy, but not with surgery. They were treated during 2007–2012 and were prospectively followed with respect to mouth opening measured in millimeters, maximal interincisal opening (MIO), and with two validated patient-reported outcome (PRO) instruments, the Gothenburg Trismus Questionnaire (GTQ) [Citation12] and the European Organization for Research and Treatment of Cancer Head and Neck Questionnaire (EORTC QLQ-HN35) [Citation13]. Patients were assessed before RT and at three, six and 12 months after RT.
Treatment and structure doses
EBRT was planned based on computed tomography (CT) imaging and was given as three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). The Eclipse™ treatment planning system was used for treatment planning (version 10.0.39, Varian Medical Systems, Palo Alto, CA, USA); no dose constraints for the mastication structures were applied. Prescribed doses were typically 1.7/2.0 Gy per fraction two/one time(s) per day, five days per week to a total dose of 64.6/68.0 Gy.
For each patient, the masseter muscles, the temporal muscles, the pterygoid muscles (medial pterygoid and lateral pterygoid), the TM joint, the ganglion pterygopalatine (ganglion PPT), and the soft palate were consistently contoured on the CT images used for treatment planning (Supplementary Figure 1, available online at http://www.informahealthcare.com) Absorbed doses for all risk structures were re-calculated according to the planned EBRT dose distribution of the original treatment plan and structure-specific dose-volume histograms (DVH) were exported. Unfortunately, reliable 3D brachytherapy dose contributions to tissues outside the tumor volume could not be estimated for most patients due to lack of image information. Instead of excluding all patients who had received brachytherapy, for each patient in question, we retained those structures where the brachytherapy absorbed dose contribution could be ignored (based on available information, Supplementary Table 1, available online at http://www.informahealthcare.com).
Evaluation of trismus and endpoints
The three investigated endpoints reflected any occurrence of trismus during the first year of follow-up. Endpoint 1 = trismus defined as MIO ≤35 mm according to Dijkstra et al. [Citation14], endpoint 2 = trismus defined as “Moderate” – “Very much” according to the five-category answering scale of the single item “Limitation in opening mouth” by GTQ [Citation12], and endpoint 3 = trismus defined as “Quite a lot” – “Much” according to the four-category answering scale of the single item “Restricted mouth opening” by EORTC QLQ-HN35 [Citation13].
Statistics
All bilateral structures were analyzed as ipsi-, contra- and bilateral structures with corresponding mean absorbed RT doses. Correlations between doses of different structures were calculated using Pearson’s correlation coefficients; comparisons between structure doses in trismus and non-trismus patients were made using Student’s t- and Wilcoxon rank-sum tests. Potential dose predictors for trismus were tested in univariable logistic regression (UVA) and in multivariable logistic regression (MVA) together with ACE-27, age, chemotherapy, height, and smoking. p < 0.05 was considered statistically significant; inclusion criterion for variables in MVA was p < 0.10. A model’s discriminative ability was assessed by the area under the receiver operating characteristic (ROC) curve (AUC). The analyses were performed using the statistical software SAS® version 9.2 (Cary, NC, USA).
Results
Patient characteristics, treatment information, and baseline outcome information for the 216 patients analyzed in this work are given in Supplementary Table 2 (available online at http://www.informahealthcare.com); detailed structure dose information in . Tumor of the oropharynx was most common (70% of patients) followed by tumors of the oral cavity (13% of patients). A majority had stage IV disease at time of diagnosis (57%); 85% of the patients had mild or no comorbidity according to ACE-27. According to the MIO criteria, 14 patients (6%) had trismus at baseline and were excluded from analyses involving the MIO endpoint. For GTQ and EORTC QLQ-HN35, the corresponding figures were 24 (11%) and 18 (8%). During the first year of follow-up, 122 patients (56%) developed trismus according to MIO, 111 (51%) according to GTQ and 66 (31%) according to EORTC QLQ-HN 35.
Table 1. Mean absorbed doses, volumes, and standard deviations for potential trismus risk structures.
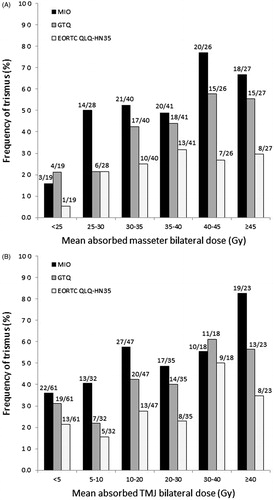
Regardless of trismus assessment approach, doses to the masseter muscle and the TM joint were significantly higher in trismus patients than in non-trismus patients (Supplementary Table 3a–c, available online at http://www.informahealthcare.com). Rates of trismus according to MIO, GTQ, and EORTC QLQ-HN35 by different dose intervals are presented for selected structures in ; increasing dose typically resulted in higher frequency of trismus. Doses of the lateral pterygoid, the temporal muscle, the TM joint, and the ganglion PPT were strongly correlated (Pearson’s correlation coefficients ≥0.80; Supplementary Table 4, available online at http://www.informahealthcare.com).
Figure 1. The relationships between dose to the masseter muscle and trismus (A) and the temporo-mandibular joint and trismus (B). Trismus according to MIO (maximal interincisal opening) ≤35 mm, GTQ (Gothenburg Trismus Questionnaire) answering categories 3–5, and EORTC QLQ-HN35 (European Organization for Research and Treatment of Cancer Head and Neck Questionnaire) answering categories 3–4.
Results from logistic regression for doses to bilateral and ipsilateral risk structures were comparable with models of similar discriminative ability ( and Supplementary Table 5a, available online at http://www.informahealthcare.com); doses to contralateral risk structures generally resulted in models with weaker discriminative ability (Supplementary Table 5b). Results for the bilateral structures are, therefore, presented in the following unless otherwise stated (cf. number of structures/patients analyzed in ; a detailed version of is also presented as Supplementary Table 6a–c, available online at http://www.informahealthcare.com).
Table 2. Logistic regression and ROC analysis of risk structure doses as predictors for trismus in head and neck cancer patients treated with radiotherapy (bilateral mean dose).
For all three endpoints, we found that dose to the masseter and the TM joint were statistically significant predictors of trismus in UVA. In MVA, smoking and height were additional factors of importance for trismus according to MIO; ACE-27 was of importance for trismus according to GTQ, and height was of importance for trismus according to EORTC QLQ-HN35. Dose to the ipsilateral masseter as predictor for trismus according to GTQ, adjusted for ACE-27, provided the model with the overall best discriminative ability for available data (N = 181; AUC = 0.78).
To investigate the relationship between the different risk structures doses as predictors for trismus, MVA with forward selection was performed (Supplementary Table 7, available online at http://www.informahealthcare.com). For MIO, the dose to the masseter muscle together with smoking was suggested as a final model. For GTQ, the dose to the masseter and ACE-27 was suggested, and for EORTC QLQ-HN35, the dose to the masseter muscle only. The ROC curves for the final models are presented in Supplementary Figure 2 (available online at http://www.informahealthcare.com); model performance is assessed in Supplementary Table 8 (available online at http://www.informahealthcare.com).
Discussion
We found that the masseter muscle mean absorbed dose was a robust predictor for both objectively determined trismus according to MIO and patient-reported trismus according to GTQ and EORTC QLQ-HN35. Clinical factors of importance were ACE-27, height, and smoking. Amongst the three investigated endpoints, trismus according to MIO provided similar results to GTQ and resulted in models with better discriminative ability than models predicting the EORTC QLQ-HN35 endpoint.
When analyzing trismus using either of the investigated methods of assessment, dose to the ipsilateral masseter muscle dose provided the model with the best discriminative ability. Other studies have also suggested that dose to the masseter is associated with a higher risk of trismus [Citation8,Citation9,Citation11]. In particular, both Lindblom et al. [Citation8] and, and more recently, Rao et al. [Citation9] found dose to the ipsilateral masseter muscle to provide superior statistics in predicting trismus (defined by MIO ≤35 mm and CTCAE grade ≥1, respectively) than dose to other investigated structures (pterygoids (both), TMJ (13), and temporalis (9)). Lindblom et al. assessed a similarly treated HNC cohort (N = 124) as investigated in this study, but with somewhat lower trismus rates (25%). Rao et al. assessed patients treated with IMRT only (N = 421) and, as can be expected, with even lower trimus rates (11%). Although there are differences in design between these studies and the present study, together these results suggest that the masseter muscle dose is a robust predictor for trismus with respect to both trismus assessment method and RT treatment approach. It is particularly interesting to note that MIO and GTQ provided very similar results for our data. Although at a GTQ-comparable trismus cutoff, results by the more commonly used EORTC QLQ-HN35 diverged substantially from both MIO and GTQ and resulted in models with weaker discriminative ability.
Strengths of this study include the large study population with prospectively collected clinical data including several different methods of assessing trismus. Numbers of missing data was low. Selection-induced problems were avoided by consecutive inclusion of patients. The 12-month follow-up inclusion criterion was established as it is unusual that patients develop trismus later than 12 months after RT completion [Citation15]. Possible confounding factors, such as dose from brachytherapy, were accounted for in our analyses and predicted trismus rates by our models were in corresponded well with the observed rates. In line with others, we based our analyses on mean absorbed structure doses as this dose metric have been reported suitable to describe relationships between various mastication structures and trismus [Citation7–11]. Acknowledging that DVH points typically are strongly correlated, it is not likely that investigating other dose or volume metrics for our purpose of identifying critical structures for radiation-induced trismus would have altered the presented results substantially. For the same reason, it is unlikely that a fractionation corrected representation of dose would have changed the presented inter-relationships between the investigated structures. As an example, R2=0.99 for masseter muscle mean absorbed and fractionation corrected dose in our data. When generalizing the results from this study, it needs to be kept in mind that our study population originates from one center, and depend on the existing treatment techniques and prescribed doses during the studied period of time. Our trismus rates were also in the high-end range compared with other studies. This is a consequence of the selected endpoint in this study (defined as the accumulation of any event during the first year after RT), which was selected to account for that some of the patients had undergone a trismus-exercise program to improve mouth opening ability [Citation16].
In conclusion, we found that predictive models based on dose to the masseter muscle were robust with respect to trismus assessment method and had the ability to well separate patients with trismus from non-trismus patients within the first year after RT. Patients receiving high radiation dose to the other investigated structures, in particular the lateral pterygoid muscle or the TM joint, can be expected to be at risk for radiation-induced trismus. Being a smoker or having other comorbidity may increase this risk, but more advanced multi-organ dose-response modeling than presented here is needed to describe these relationships in detail. Accumulated evidence, however, still suggest that the masseter muscle is a suitable candidate for trismus sparing techniques in cases where mastication structure doses can be lowered without jeopardizing tumor control.
Department of Otorhinolaryngology, Head and Neck Surgery, Institute of Clinical Sciences, Sahlgrenska Academy at the University of Gothenburg, Sahlgrenska University Hospital, Gothenburg, Sweden
Caroline E. Olsson
Department of Radiation Physics, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
[email protected] Pettersson
Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
Mia Johansson and Hedda Haugen
Department of Oncology, Institute of Clinical Sciences, Sahlgrenska Academy at the University of Gothenburg, Sahlgrenska University Hospital, Gothenburg, Sweden
Ulrica Wilder´ng and Gunnar Steineck
Division of Clinical Cancer Epidemiology, Department of Oncology, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
Supplementary_material.docx
Download MS Word (270.6 KB)Acknowledgments
This study was financially supported by the Swedish Cancer Society; the Research and Development Council, West Region (Sweden) County, the Assar Gabrielsson Foundation and the King Gurav V Jubilee Clinic Cancer Foundation in Göteborg, the Swedish Society for Medical Research (SSMF), the Kamprad Family Foundation for Entrepreneurship Research & Charity, and the Medical Faculty of Gothenburg University Sweden. The authors would like to acknowledge Inger Nilsson, senior neuroradiologist at the Sahlgrenska University Hospital, for guidance and support with defining anatomical boundaries, and research assistant Alireza Sadeghi for delineation work.
Disclosure statement
No conflicts of interest exist for any of the authors involved.
References
- Abendstein H, Nordgren M, Boysen M, Jannert M, Silander E, Ahlner-Elmqvist M, et al. Quality of life and head and neck cancer: A 5 year prospective study. Laryngoscope 2005;115:2183–92.
- Johnson J, van As-Brooks CJ, Fagerberg-Mohlin B, Finizia C. Trismus in head and neck cancer patients in Sweden: Incidence and risk factors. Med Sci Monit 2010;16:CR278–82.
- Pauli N, Johnson J, Finizia C, Andrell P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol 2013;52:1137–45.
- Louise Kent M, Brennan MT, Noll JL, Fox PC, Burri SH, Hunter JC, et al. Radiation-induced trismus in head and neck cancer patients. Support Care Cancer 2008;16:305–9.
- Weber C, Dommerich S, Pau HW, Kramp B. Limited mouth opening after primary therapy of head and neck cancer. Oral and Maxillofacial Surgery 2010;14:169–73.
- Wetzels JW, Merkx MA, de Haan AF, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: A 1-year prospective study. Head Neck 2014;36:1754–62.
- Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: A prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:365–73.
- Lindblom U, Gärskog O, Kjellén E, Laurell G, Levring Jäghagen E, Wahlberg P, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncologica 2014;53:620–7.
- Rao SD, Saleh ZH, Setton J, Tam M, McBride SM, Riaz N, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol 2016;55:99–104.
- Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, de Kruijf W, et al. Trismus in patients with oropharyngeal cancer: Relationship with dose in structures of mastication apparatus. Head Neck 2008;30:622–30.
- van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CR, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol 2013;106:364–9.
- Johnson J, Carlsson S, Johansson M, Pauli N, Ryden A, Fagerberg-Mohlin B, et al. Development and validation of the Gothenburg Trismus Questionnaire (GTQ). Oral Oncol 2012;48:730–6.
- Bjordal K, Ahlner-Elmqvist M, Tollesson E, Jensen AB, Razavi D, Maher EJ, et al. Development of a European Organization for Research and Treatment of Cancer (Eortc) Questionnaire module to be used in quality of life assessments in head and neck cancer patients. Acta Oncologica 1994;33:879–85.
- Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg 2006;35:337–42.
- Wang C-J, Huang E-Y, Hsu H-C, Chen H-C, Fang F-M, Hsiung C-Y. The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope 2005;115:1458–60.
- Pauli N, Fagerberg-Mohlin B, Andrell P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol 2013;
