Abstract
Background To investigate potential changes in perceptual, acoustic and patient-reported outcomes over 12 months for laryngeal cancer patients treated with radiotherapy.
Material and methods A total of 40 patients with Tis-T3 laryngeal cancer treated with curative intent by radiotherapy were included in this prospective longitudinal descriptive study. Patients were followed pre-radiotherapy, one month, six months and 12 months post-radiotherapy, where voice recordings and patient-reported outcome instruments (European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core30, Head and Neck35, Swedish Self-Evaluation of Communication Experiences after Laryngeal Cancer) were completed at each appointment. Perceptual analysis, using the Grade-Roughness-Breathiness-Asthenia-Strain scale and vocal fry parameters, and acoustic measures including harmonics-to-noise ratio (HNR), jitter, shimmer and mean spoken fundamental frequency (MSFF) were produced from voice recordings.
Results All patients presented with dysphonic voices pre-radiotherapy, where 95% demonstrated some degree of vocal roughness. This variable improved significantly immediately post-radiotherapy, however, then deteriorated again between six and 12 months. Vocal fry also increased significantly at 12 months. Acoustic measures were abnormal pre- and post-treatment with no significant change noted except for MSFF, which lowered significantly by 12 months. Health-related quality of life (HRQL) deteriorated post-radiotherapy but returned to pretreatment levels by 12 months.
Conclusion By 12 months, most perceptual, acoustic, patient-reported voice and HRQL outcomes for laryngeal cancer patients treated by radiotherapy had showed no significant improvements compared to pretreatment function. Further studies are required to investigate potential benefits of voice rehabilitation following radiotherapy.
Laryngeal cancer is the second most common head and neck (H&N) cancer worldwide with 157 000 new cases diagnosed in 2012 [Citation1]. Early stage disease is managed with organ preserving treatments, such as surgery or radiotherapy, which despite being effective for cancer control are not always synonymous with function preservation. Both the tumor and subsequent oncologic treatment affect voice, vocal communication and health-related quality of life (HRQL) in this population [Citation2–5]. Hence, fully understanding the impact of laryngeal cancer on vocal function requires documenting both the presenting vocal behavior at time of diagnosis and changes over time in response to treatment effects.
Any assessment battery should also mirror the multidimensional nature of voice and its effect on the individual, thereby including perceptual and instrumental voice measures as well as patient-reported outcomes (PRO) [Citation4]. Studies using perceptual voice analysis have found that early laryngeal cancer patients often present with a mild-moderate dysphonia post-radiotherapy [Citation4]. These dysphonic changes have been shown to persist up to five years post-radiotherapy in 50–95% of patients, typically characterized by a rough, breathy and strained quality [Citation6]. Acoustically, spontaneous recovery post-radiotherapy with return to pretreatment values at 12 months following treatment completion has been shown. The voice, however, still remains pathological as indicated by abnormal perturbation measures [Citation4,Citation7]. In long-term follow-up studies, some acoustic measures normalize, whereas others remain pathological [Citation8,Citation9].
PRO, including communicative ability and HRQL, are essential components in the multidimensional assessment post-radiotherapy. However, as HRQL instruments often contain only single statements regarding voice and communication, instruments specifically addressing communicative ability are important additions to assess rehabilitative needs and psychosocial outcomes in this patient population [Citation2]. Prior studies have shown that at diagnosis, general HRQL and communication function for H&N cancer patients are significantly reduced compared to the normal population [Citation10]. Data supports declines immediately post-treatment but returns to pretreatment levels, with the exception of treatment-specific side effects, such as salivary function, which deteriorate further [Citation5].
Research within laryngeal cancer management has evolved from focusing purely on survival rates and tumor control to incorporating functional and HRQL outcomes. Most current studies of functional voice outcomes, however, are retrospective in design, do not use combinations of multidimensional assessment tools nor do they include pretreatment measures or normative comparison [Citation3]. Hence, this study aims to report longitudinal results from systematically collected data in order to more fully understand the nature of the vocal deficits, voice changes over time and impact on HRQL outcomes for laryngeal cancer patients treated with radiotherapy. This information is needed to help inform the post-treatment functional rehabilitation, which has been advocated.
Material and methods
Study population
Data were collected as part of a larger, prospective randomized controlled trial at Sahlgrenska University Hospital, Gothenburg, Sweden. All patients diagnosed with laryngeal cancer treated curatively with radiotherapy ± chemotherapy from March 2000 to December 2011 were eligible for inclusion. Inclusion criterion was Swedish language competency to independently answer the questionnaires and partake in rehabilitation. Exclusion criteria included cognitive impairment hampering participation in rehabilitative exercises. Eighty-nine patients were available for randomization in two groups: a study group receiving vocal rehabilitation and a control group. Forty of these patients constituted the control group, which is the cohort population analyzed in this study. Comorbidity was measured using the Adult Comorbidity Evaluation-27.
Study design
In this prospective descriptive longitudinal study, patients were followed at four time points: pretreatment, one month post-radiotherapy, six months post-radiotherapy and 12 months post-radiotherapy. At each visit, the voice was recorded and PRO instruments, as described below, answered. One patient was excluded from acoustic analysis as there was only data for one time point. One patient was excluded from PRO analyses for the same reason.
Oncologic treatment
All patients received radiotherapy with curative intent according to regional treatment guidelines and was either hyperfractionated (n = 11) or conventional (n = 29). Conventionally fractionated radiotherapy was administered once daily in 2–2.4 Gy fractions totaling 62.4–68 Gy, whereas the hyperfractionated radiotherapy consisted of 1.7 Gy doses given twice daily to a total of 64.6 Gy. Most patients with T2-T3 tumors also received irradiation to the lymph nodes to a total of 40.8 Gy (1.7 Gy twice a day, five days a week n = 11) or 46 Gy (2.0 Gy once a day, five days a week n = 4). One patient with a T3-tumor received induction chemotherapy (trapoxin).
Voice recordings
Voice recordings included reading of a standard passage and the maximum sustained vowel/a/repeated three times. A headset microphone (Sennheiser MKE 2-p) was set at a distance of 12 cm from the corner of the mouth. Recordings were made at a sampling frequency of 44.1 kHz with a Panasonic Professional Digital Audio Tape (DAT) Recorder SV-3800. Prior to analysis, all recordings were transferred from a DAT to a computer hard drive as an audio file (.wav) using the program Swell Soundfile Editor, version 4.5 (Saven Hightech).
For perceptual rating, excerpts from the voice recordings were cut using Swell Soundfile Editor (4.5). These excerpts, i.e. rating samples, included the first two sentences of the standard passage and the second recorded prolonged vowel/a/and were saved as audio files (.wav). Samples were compiled from each patient across each of the four time points (one file/person/time point = 156). Nine patients missed one or two voice recordings, totaling 14 (9%) missed recordings. Missing values were imputed using the Last Observation Carried Forward method. Twenty percent of samples were randomly reduplicated for intra-rater reliability calculations. All samples were then randomly compiled with anchor samples interspersed, at every 20 voice samples, into the final rating file for perceptual analyses.
Perceptual analyses
Perceptual ratings were conducted by two speech language pathologists. All raters attended a half-day’s consensus training. The raters were blinded to patient status and voice sample information. The rating protocol used the GRBAS scale [Citation11] which consists of five voice qualities: grade, roughness, breathiness, asthenia, and strain. Each voice quality is rated on a four-point scale, where 0 = normal, 1 = mildly impaired, 2 = moderately impaired and 3 = severely impaired. The vocal fry parameter (rated on a four-point scale mirroring the GRBAS) was added to the rating protocol as it has also been reported in irradiated voices [Citation12,Citation13].
Acoustic analyses
Voices were analyzed using Voxalys, a plugin program to Praat. Jitter, shimmer (perturbation measures which refer to the acoustic signal’s cycle-to-cycle variation in the fundamental frequency and amplitude, respectively) and harmonics-to-noise ratio (HNR) values were analyzed from two seconds of the middle of the second sustained vowel/a/. Mean speaking fundamental frequency (MSFF) was measured from reading of the standard passage.
Pro
Swedish Self-Evaluation of Communication Experiences after Laryngeal Cancer (S-SECEL)
The S-SECEL consists of 35 items covering communication experiences and dysfunction in laryngeal cancer patients. It has proven reliability, convergent and discriminant validity and satisfactory internal consistency [Citation14]. S-SECEL consists of three domains (General, Environmental and Attitudinal) and a Total score domain, totaling 34 questions and one separate question not included in the scoring. Each item is rated on a four-point categorical scale ranging from 0 (never) to 3 (always) and recalls the last 30 days. The cumulative subscale scores range from 0 to 15 for General, 0–42 for Environmental, 0–45 for Attitudinal and 0–102 for Total, where a higher score indicates greater perceived communicative dysfunction [Citation14].
EORTC QLQ-C30 and EORTC QLQ-H&N35
The European Organization for Research and Treatment of Cancer (EORTC) has developed self-administered questionnaires specifically investigating the HRQL for cancer patients. The Core-30 questionnaire (EORTC QLQ-C30 version 3.0) consists of 30 items addressing patient functioning and symptomatology over the last week. The additional 35 items in the head and neck module (H&N35) cover symptoms specific to H&N cancer and its treatments. Scores for each item or domain range from 0 to 100. On functioning domains and HRQL domains, a higher score indicates better functioning whereas on symptom domains or single items higher scores represent increasing symptom burden. For the EORTC QLQ-C30, a difference of 10 points may be considered a clinically significant difference, and this criterion is frequently used when interpreting results in the H&N35 as well. Only domains that may influence voice and/or communication from these tools were used in the current study.
Statistical analyses
The SPSS version 20.0 for Mac and SAS version 9.2 for PC were used for analyses. Mean values, standard deviation and 95% confidence intervals (CI) were used for descriptive purposes. Non-parametric tests were used due to data being of non-normal distribution. All tests were two-tailed and the significance level was set at p < 0.05. Repeated measures tests for overall significant differences within groups, over time, were conducted using Friedman’s test and post-hoc analyses performed where significance was found. Paired intra-group comparisons over time were carried out with Wilcoxon Signed Rank test for continuous variables and Sign test for ordered categorical variables. Corresponding tests used for between group comparisons were the Mann-Whitney U-test and Mantel-Haenszel χ2 exact test, respectively. Magnitude of group differences were further analyzed using effect sizes (ES). ES of within-group change was calculated as mean score at follow-up – mean score at pretreatment divided by the standard deviation of change. ES was interpreted according to Cohen’s standard criteria where size is classified as trivial (0–<0.2), small (0.2–<0.5), moderate (0.5–<0.8) or large (≥0.8). This method complements standard significance testing and yields standardized effect levels regardless of sample size and scaling properties.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Gothenburg, Sweden. Parts of this study population have been described previously [Citation15].
Results
Clinical characteristics for the study population and subdivisions depending on tumor stages T1 and T2 are shown in .
Table 1. Clinical characteristics at pre-radiotherapy.
Perceptual
Both inter- and intra-rater reliability were calculated for the two raters using percent exact agreement (PEA), percent close agreement (PCA: one-point difference) and Weighted Kappa. Inter-rater reliability revealed a PEA of 59%, and PCA of 94%. Weighted Kappa was calculated at 0.55, indicating a moderate agreement. Intra-rater reliability revealed PEA 69%, and PCA 96% and a Weighted Kappa of 0.70, indicating a substantial intra-rater agreement. Where ratings differed between the two clinicians, a third clinician rated the parameter and consensus rating (two of three) was used in the analysis.
The variable grade did not change significantly between the four time points. Pretreatment, all patients presented with some degree of dysphonia, which did not change significantly post-radiotherapy (). The voice quality of roughness, however, did change significantly, whereby pretreatment, 95% of patients presented with some degree of roughness, which improved one month post-radiotherapy (p = 0.04). Between six and 12 months post-radiotherapy roughness deteriorated significantly to again present with a rough quality in 95% patients (p = 0.02). The qualities of breathiness, asthenia and strain, which pretreatment were observed to be present in 61%, 8% and 64% of patient, respectively, showed no significant change over time. Vocal fry demonstrated a statistically significant deterioration from post-radiotherapy to 12 months (p = 0.02).
Table 2. Results of grade, roughness, asthenia, strain and vocal fry at pretreatment and follow-up (n = 39).
Acoustic
The acoustic analyses of HNR, jitter and shimmer demonstrated no significant change over time (). Only MSFF for males and females showed statistically significant changes over time (p < 0.001 and p = 0.025, respectively) (). For males (n = 35), the median value at the first two time points presented with a relatively high MSFF (pitch) pretreatment compared with published norms [Citation16]. However, this decreased to a lower pitch at the six and 12 months post-radiotherapy. Females (n = 4), however, presented with pitches lower than published norms [Citation16] and showed a similar pattern as males of further lowering of pitch over time [Citation15].
Figure 1. Mean spoken fundamental frequency (MSFF) for A) males (n = 35) and B) females (n = 4) at pretreatment and follow-up. The line inside the box represents the median, the dot represents the mean value and the upper and lower border are the upper and lower quartiles. §MSFF (healthy reference values).
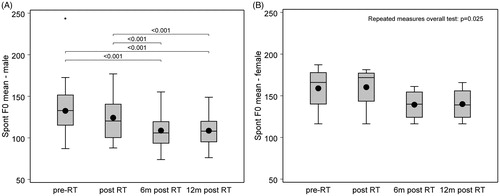
Table 3. Mean values (SD) (95% CI) for acoustic measures pretreatment and follow-up time points.
S-SECEL
S-SECEL results are shown in . Total scores pre-radiotherapy indicated patients perceived a degree of impaired function. A statistically significant improvement was seen between pre- and one month post-radiotherapy in Total score (p = 0.046, ES = 0.24). No other domain showed significant change over time and had ES of very weak magnitude.
Table 4. Longitudinal S-SECEL scores presented as mean (SD) (95% CI) values including p-values and effect sizes for changes at follow-up compared to pretreatment.
HRQL: EORTC QLQ-C30 and EORTC QLQ-H&N35
Selected voice impacting EORTC parameters are presented in Supplementary available online at http://www.informahealthcare.com). At one month post-radiotherapy, patients reported trends of deterioration in all domains (except Emotional functioning) of which changes in domains Physical functioning, Fatigue, Dry mouth and Sticky saliva were statistically significant with ES ranging from moderate to borderline strong. At six months, nearly all domain scores were similar to pretreatment again and remained unchanged at 12 months. The domains Sticky saliva and Dry mouth, however, demonstrated constant clinically and statistically significant deteriorations at all time points post-radiotherapy (ES = 0.38–0.60).
T1 and T2 subdivisions
To ensure group patterns were not unduly influences by larger tumor size, exploratory analysis was performed by subdividing the larger cohort according to tumor stage, T1 and T2 (). More patients received hyperfractionated and adjuvant radiotherapy in the T2 subgroup. There were also more supraglottic and subglottic tumors in the T2 subgroup (not statistically significant). Only vocal fry in the perceptual analyses differed significantly between T1 and T2 tumors whereby, pretreatment, the T2 cohort presented with more moderate-severe vocal fry (p = 0.03, data not shown). No statistically significant difference between the two cohorts was found regarding acoustic data.
For S-SECEL, the T1- and T2-subpopulations demonstrated no statistically significant changes in any domain over time. According to EORTC QLQ-C30 and H&N35, the T2-subpopulation reported trends of inferior function and increased symptom burden pretreatment across all domains compared to the T1-cohort. The main statistically significant change was seen in the Speech domain, where T2-patients had significantly higher scores at pretreatment compared to the T1-cohort (47 vs. 29 p). T2-patients also reported clinically and statistically significant post-radiotherapy improvements in the Speech domain, resulting in no inter-group score difference at 12 months (data not shown).
Discussion
This study is one of few that reports longitudinal voice outcome combining perceptual, acoustic and PRO with HRQL measures in patients treated by radiotherapy for laryngeal cancer. Results indicate that vocal function at 12 months was comparable to pretreatment levels. Yet, these remain abnormal.
When examining perceptual voice progression, no significant change was seen at 12 months post-radiotherapy compared to pretreatment in any of the GRBAS or vocal fry parameters. However, pretreatment findings were far from normal with roughness, breathiness and strain presenting in 95%, 61% and 64%, respectively. Similar findings were presented by Niedzielska et al., [Citation9] in which all of their 45 males with T1-T2 disease presented with pathological voices at pretreatment, which had not completely normalized 1–3 years later. Furthermore, reports indicate that these abnormal perceptual qualities persist up to 10 years post-treatment [Citation13,Citation17].
Similar results are found acoustically, where jitter and shimmer values are higher (abnormal) at all time points compared to published Praat reference data (jitter and shimmer normal reference ranges being 0.30–0.67% and 0.31–0.32 dB, respectively) [Citation15]. Pathological acoustic values pre-radiotherapy have also been reported by others, followed by trends of improvement post-radiotherapy [Citation4]. Surprisingly, our parameters did not change significantly throughout the study period and, similar to perceptual results, no statistically significant difference from pretreatment to study endpoint was observed. This discrepancy between studies may be explained by different voice analysis software being used in different studies [Citation18].
The EORTC and S-SECEL showed similar trends, where patients reported scores similar to pretreatment at six and 12 months, except for treatment-specific symptoms, in line with recent results reported by a systematic review for H&N cancer by Klein et al. (2014) [Citation5]. Nevertheless, pretreatment scores reported in this study imply inferior outcomes when compared to published normative values [Citation10,Citation19].
The vocal parameters which demonstrated the greatest amount of change over time were roughness, vocal fry and MSFF. Roughness improved from pretreatment to immediately post-radiotherapy and then deteriorated at six and 12 months. The initial improvement was also noted subjectively (H&N35 Speech, S-SECEL) and is most likely explained by removal of tumor burden [Citation3]. Roughness is the perception of irregular glottic pulses resulting from unsynchronized vocal fold vibration [Citation20]. Hence, the noted late deterioration could be attributed to the delayed radiotherapy effects, which cause altered microcirculation, acute oxidative responses, neuromuscular fold weakness, fibrosis, inflammation and edema. Other subsequent structural effects reported include impaired irregular vocal cord mobility, glottic incompetence, pooling of sticky secretions and recruitment of ventricular folds [Citation21]. As fundamental frequency is affected by mass, elasticity, length and compliance of vocal cords, it follows that MSFF reduces post-radiotherapy for abovementioned reasons [Citation22]. Interestingly, MSFF was the only acoustic variable that significantly changed during the study by reducing from pretreatment to endpoint – a reduction, which can contribute to rough sounding voices [Citation22]. An additional explanation for deterioration in roughness is the reported xerostomia post-radiotherapy that persists up to 12 months. Studies have suggested that throat dryness and thickened secretions negatively influences voice quality in laryngeal and H&N cancer [Citation17,Citation19]. Verdolini et al. [Citation23] further demonstrated the effects of vocal dryness by observing an increased phonation threshold pressure after inducing systemic dehydration. Given this poorer phonation ability combined with vocal fold dryness and sticky secretions, the voice quality of vocal fry should also increase, which was observed in this study. Vocal fry is caused by intermittent energy packets below 70 Hz [Citation20] and if present can also lower MSFF further. Its presence can further increase the perception of a rough voice as the two can be difficult to distinguish perceptually.
Clinical implications
Clearly, although voice, communication and HRQL mostly return to pretreatment levels, this level of function is not normal. Patients with laryngeal cancer prioritize the importance of speech and voice following radiotherapy and subnormal function negatively influences social activities [Citation2,Citation19]. Consequently, we advocate voice rehabilitation, as do others [Citation17,Citation24], yet which voice qualities should be targeted and when are rarely discussed. Based on the study findings, vocal rehabilitation needs to target the voice qualities of roughness, breathiness and strain. Earlier implementation may help to hinder effects of irradiation fibrosis progression, which has been shown to cause more collagen deposition and severe morphological changes after 12–24 months [Citation17], and decrease risk of developing maladaptive compensatory vocal behaviors [Citation4]. The majority of study participants were smokers at diagnosis, which has been reported as a risk factor for deteriorating voice quality post-radiotherapy and smoking cessation should be advocated [Citation17]. Declines in HRQL, functioning and increased symptom burden observed immediately post-radiotherapy may impact patient engagement in voice therapy. However, as these were all found to improve again by six months, patients will have greater capacity, in time, to take on more active rehabilitation.
Nevertheless, voice rehabilitation is unlikely appropriate for all patients and therapists should consider specific needs, vocal use socially and professionally and HRQL effect. Similar to others, this study found that although T2 tumors reported more problems pretreatment, they did not remain inferior at endpoint [Citation25] and should perhaps not be the guiding factor. This study also highlighted that female MSFF is lower than normative values () suggesting a potential risk group that may benefit from voice rehabilitation, although it should be noted that all women were smokers at the start of the study. Nonetheless, further research is required to elucidate potential patient groups who would benefit from voice rehabilitation.
The strengths of this study include its prospective longitudinal design and multimodal voice and PRO assessment. It is limited by the lack of a vocally healthy control group and laryngeal videostroboscopic measurements to assess voice function. Also, the moderate agreement for inter-rater reliability is limiting, which may be explained by the mixed and complex dysphonia that is characteristic of the irradiated voice.
Conclusion
Laryngeal cancer patients managed with organ preservation treatment (radiotherapy), show no significant differences for the majority of voice, communication and HRQL outcomes from pretreatment to 12 months, and continue to present with abnormal voice qualities at 12 month. The impact of rehabilitation on these persistent deficits has received little attention in the literature and randomized studies investigating the potential positive effects of voice rehabilitation on voice function and HRQL are needed.
Supplementary_table_1.docx
Download MS Word (100 KB)Acknowledgements
Gothenburg Society of Medical Physicians (GLS), Swedish Cancer Society, Sahlgrenska University Foundation, Assar Gabrielsson Foundation, The Laryng Foundation, Lions Cancer Foundation West, the Research and Development Council (FoU), Västra Götaland County and the Medical Faculty of Gothenburg University, Sweden.
Disclosure statement
No actual or potential conflicts of interest exist.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No.11. Lyon, France: International Agency for Research on Cancer; 2013.
- Peeters AJ, van Gogh CD, Goor KM, Verdonck-de Leeuw IM, Langendijk JA, Mahieu HF. Health status and voice outcome after treatment for T1a glottic carcinoma. Eur Arch Oto-Rhino-Laryngol 2004;261:534–40.
- Jacobi I, van der Molen L, Huiskens H, van Rossum MA, Hilgers FJ. Voice and speech outcomes of chemoradiation for advanced head and neck cancer: a systematic review. Eur Arch Oto-Rhino-Laryngol 2010;267:1495–505.
- Bibby JR, Cotton SM, Perry A, Corry JF. Voice outcomes after radiotherapy treatment for early glottic cancer: assessment using multidimensional tools. Head Neck 2008;30:600–10.
- Klein J, Livergant J, Ringash J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: a systematic review. Oral Oncol 2014;50: 254–62.
- Sjogren EV, van Rossum MA, Langeveld TP, Voerman MS, van de Kamp VA, Friebel MO, et al. Voice outcome in T1a midcord glottic carcinoma: laser surgery vs radiotherapy. Arch Otolaryngol Head Neck Surg 2008;134:965–72.
- van Gogh CD, Verdonck-de Leeuw IM, Wedler-Peeters J, Langendijk JA, Mahieu HF. Prospective evaluation of voice outcome during the first two years in male patients treated by radiotherapy or laser surgery for T1a glottic carcinoma. Eur Arch Oto-Rhino-Laryngol 2012;269:1647–52.
- Krengli M, Policarpo M, Manfredda I, Aluffi P, Gambaro G, Panella M, et al. Voice quality after treatment for T1a glottic carcinoma–radiotherapy versus laser cordectomy. Acta Oncol 2004;43:284–9.
- Niedzielska G, Niedzielski A, Toman D. Voice after radiotherapy of the larynx carcinoma. Radiother Oncol J Eur Soc Therapeutic Radiol Oncol 2010;97:276–80.
- Hammerlid E, Taft C. Health-related quality of life in long-term head and neck cancer survivors: a comparison with general population norms. Br J Cancer 2001;84:149–56.
- Hirano M. Psycho-acoustic evaluation of voice. In: Disorders of Human Communication, 5, Clinical examination of voice. New York, Wien.: Springer-Verlag; 1981.
- van Gogh CD, Verdonck-de Leeuw IM, Boon-Kamma BA, Rinkel RN, de Bruin MD, Langendijk JA, et al. The efficacy of voice therapy in patients after treatment for early glottic carcinoma. Cancer 2006;106:95–105.
- Morgan DA, Robinson HF, Marsh L, Bradley PJ. Vocal quality 10 years after radiotherapy for early glottic cancer. Clin Radiol 1988;39:295–6.
- Finizia C, Palme C, Bergman B. A longitudinal study of the Swedish Self-Evaluation of Communication Experiences after Laryngeal Cancer questionnaire in patients treated for laryngeal cancer. Acta Oncol 2002;41:262–8.
- Tuomi L, Karlsson T, Johansson M, Finizia C. Health-related quality of life and voice following radiotherapy for laryngeal cancer - a comparison between glottic and supraglottic tumours. Acta Oncol 2015;54:73–9.
- Lindblad P. Rösten. Lund: Studentlitteratur; 1992.
- Hocevar-Boltezar I, Zargi M, Strojan P. Risk factors for voice quality after radiotherapy for early glottic cancer. Radiother Oncol J Eur Soc Therapeutic Radiol Oncol 2009;93:524–9.
- Maryn Y, Corthals P, De Bodt M, Van Cauwenberge P, Deliyski D. Perturbation Measures of Voice: a Comparative Study between Multi-Dimensional Voice Program and Praat. Folia Phoniatr Logop 2009;61:217–26.
- Roh JL, Kim AY, Cho MJ. Xerostomia following radiotherapy of the head and neck affects vocal function. J Clin Oncol 2005;23: 3016–23.
- National Center for Voice and Speech. 2014. [http://www.ncvs.org/]
- Meleca RJ, Dworkin JP, Kewson DT, Stachler RJ, Hill SL. Functional outcomes following nonsurgical treatment for advanced-stage laryngeal carcinoma. Laryngoscope 2003;113:720–8.
- Davies SB. Aoustic characteristics of normal and pathological voices. Haskins Laboratories: Statis Rep. on Speech Res. 1978;54: 133–64.
- Verdolini K, Min Y, Titze IR, Lemke J, Brown K, van Mersbergen M, et al. Biological mechanisms underlying voice changes due to dehydration. J Speech Lang Hear Res 2002;45:268–81.
- Roh JL, Kim DH, Kim SY, Park CI. Quality of life and voice in patients after laser cordectomy for Tis and T1 glottic carcinomas. Head Neck 2007;29:1010–16.
- Agarwal JP, Baccher GK, Waghmare CM, Mallick I, Ghosh-Laskar S, Budrukkar A, et al. Factors affecting the quality of voice in the early glottic cancer treated with radiotherapy. Radiother Oncol 2009;90:177–82.
- Ferrand C. Harmonics-to-noise ratio: an index of vocal ageing. J Voice 2002;16: 480–7.
