Abstract
Background and purpose Evidence suggests that distinct biologic phenomenon produce different patterns of distant metastatic (DM) failures. We attempted to identify prognostically poor sites of first DM and to define factors predictive of their development. Methods and materials A total of 1074 patients treated with ≥60 Gy definitive radiation for initially non-metastatic non-small cell lung cancer (NSCLC) were analyzed. Uni- and multivariate Cox regression was utilized to associate clinical factors with DM site, and metastatic site with overall survival (OS). To account for competing events, multivariate Fine and Gray regression was utilized to identify treatment and disease factors predictive of site-specific metastases. Results Sites of first DM associated with worse survival were liver (median OS: 5 months after DM) and bone (median OS: 6.7 months after DM). Multivariate regression identified non-squamous histology to be associated with first DM within the liver (HR = 2.04, 95% CI 1.16–3.60, p = 0.01), while delay between diagnosis and RT (third vs. first tertile: HR = 2.3, 95% CI 1.26–4.21, p = 0.007) in addition to advanced stage (stage III vs. II/I: HR = 2.37, 95% CI 1.11–5.06, p = 0.03) were associated with first DM within bone. Conclusions Liver and bone as site of first DM is associated with worse prognosis and are predicted by different disease and treatment factors.
Metastatic disease represents a common mechanism of failure after definitive treatment for non-small cell lung cancer (NSCLC), with reports ranging from 33% to 59% distant failure rates [Citation1–3]. Recent investigation into the biology underlying distant metastases (DM) suggests that distinct molecular phenotypes are associated with specific sites of metastatic development [Citation4,Citation5]. The “seed and soil” analogy proposed by Paget provides a useful framework for this phenomenon [Citation6]. Specifically, distinct alterations within a tumor or tumor sub-clone endow circulating cancer cells (or “seeds”) with the molecular machinery to survive, invade, and proliferate within a subset of preferred distant microenvironments (or “soil”) [Citation4–6]. As a clinical corollary, it is feasible that clinical tumor and treatment characteristics reflect propensities for different metastatic sites. Investigation into this hypothesis has identified histology and advanced initial stage to be associated with DM [Citation2,Citation7,Citation8]. In addition, other studies have also associated DM location and initial lesion number with clinical trajectory [Citation9,Citation10].
Although these data have provided insight into the underlying biology of metastatic disease and its clinical behavior in diseases including NSCLC, few modern studies have thoroughly investigated this phenomenon in a spectrum of metastatic sites. The behavior of metastatic disease is of particular contemporary importance given the recent trends to treat limited metastatic disease with definitive intent [Citation4,Citation10–12]. We therefore sought to identify first DM sites with worse prognosis and then to describe tumor and treatment characteristics predictive of site-specific DM in NSCLC patients treated with definitive radiation therapy (RT).
Material and methods
Institutional Review Board approval was granted for this retrospective study and informed consent was waived. Study subjects were selected from a database of consecutive NSCLC patients treated with definitive RT at a single tertiary cancer center. Inclusion criteria were receipt of definitive ≥60 Gy RT with curative intent and pathologic confirmation of NSCLC. Exclusion criteria were enrollment in a protocol that excludes outcome assessment, initial definitive stereotactic ablative RT, initial presentation with recurrent or metastatic disease, and initial treatment with adjuvant RT after primary surgical intervention. NSCLC pathologies were limited to adenocarcinoma, squamous, NSCLC not otherwise specified (NOS), and large cell. Patient confidentiality was maintained in accordance with the Health Insurance Portability and Accountability Act.
Surveillance for distant metastasis
Routine patient visits including history and physical examinations with routine imaging were conducted at least once before RT, every 3–4 months after RT completion for the first 2–3 years, twice a year until five years, and annually thereafter. Routine imaging consisted of either chest computed tomography (CT), which includes evaluation of the liver and adrenal glands, or positron emission tomography (PET)/CT. Data on the modality of pretreatment imaging was available among 753 patients, of which 674 (90%) had one PET/CT scan prior initiation of radiation. In the event of concerning symptoms or laboratory values, additional imaging including brain magnetic resonance imaging (MRI), x-ray, ultrasound, or abdominal/pelvic CT scans was obtained.
An organ system was considered involved with metastasis if imaging revealed at least one lesion with unequivocal metastatic characteristics. Records of pathologic confirmation were reviewed to verify metastatic involvement when available. Distant sites of first metastasis were classified as lung, brain, bone, adrenal, or liver. First DM was classified as “other” if occurring in a site outside of those listed previously. Lung metastases were discerned from locoregional relapses per the judgment of a reviewing radiation oncologist based on imaging and pathology reports. Lung metastases were generally recorded when evaluation revealed pleural metastasis, cytologically confirmed pleural effusion, and/or a metastatic nodule outside of the original involved lobe. All sites identified with additional evaluation within one month of the identification of first DM were considered simultaneous first DM sites.
Statistical methods
There were two primary objectives: First, to determine whether specific sites of first DM predict for worse overall survival (OS); second, to identify disease and treatment factors associated with development of first DM in prognostically unfavorable sites.
To assess the first objective, uni- and multivariate Cox regression were conducted to associate site of first DM with OS. To assess the variations in death rate with time, kernel-based estimates of the hazards of death plotted over time (as described in [Citation13]). To analyze the second objective, Fine and Gray multivariate competing risk regression was utilized to identify factors predictive of first DM [Citation14,Citation15]. Competing events in this model were death and development of first DM outside of the DM site of consideration. For example, if analyzing factors predictive of first DM within the liver, death without DM or first DM in the lung, bone, brain, adrenal, or other site were considered competing risks. All p-values were two-sided with significance taken at p < 0.05. Analyzes were conducted with SAS v9.3 (SAS Institute, Cary, NC, USA) and TIBCO Spotfire S+ (TIBCO Software Inc, Palo Alto, CA, USA).
Results
Patient characteristics
Between December 1998 and June 2012 a total of 2482 consecutive NSCLC patients were treated with radiation and entered into our institutional database, of which 1074 patients (43%) met study inclusion criteria. Baseline treatment and demographics of these patients are presented in . The majority of patients were male (54%) with T2-3 (58%) N2-3 (68%) disease with adenocarcinoma (41%) or squamous (40%) histology treated with concurrent (71%) chemoradiation. Radiation was primarily administered as either three-dimensional conformal RT (3D-CRT; 43%) or intensity modulated RT (IMRT; 40%). A minority of patients received induction (37%) and/or adjuvant (17%) chemotherapy. The identity of the most common concurrent chemotherapy agents are presented in Supplementary Table 1 (available online at http://www.informahealthcare.com).
Table 1. Baseline patient characteristics.
First distant metastatic site and survival
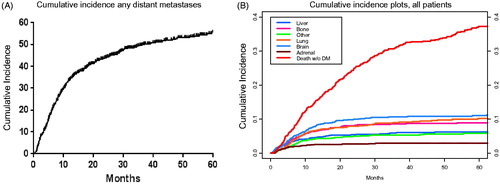
Among all patients, there were 456 instances (42%) of DM that occurred at a median of 6.9 months (range 0.1–89.8 months) after radiation. In decreasing order of frequency brain, lung, bone, liver, and adrenal as first sites of metastasis occurred in 146 (14%), 125 (12%), 98 (9%), 63 (6%), and 49 (5%) patients, respectively (). Cumulative incidence of different sites of first DM in relation to death without DM is plotted in . Among all patients, median survival was 24 months after radiation completion, with two- and five-year OS rates of 50% and 23%, respectively. In addition, 309 (29%) patients experienced locoregional failure. Among patients who developed DM, there were 361 (79%) deaths (median survival: 9.0 months after DM) over a median follow-up time of 7.6 months (range 0.1–55.6 months) after first DM. A sensitivity analysis conducted on the 762 patients who received concurrent chemotherapy identified a similar rate of DM (359 patients, 47%). With the same ordering of first metastatic sites in terms of frequency: brain (16%), lung (12%), bone (9%), liver (7%), and adrenal (5%). Similarly, among patients who received concurrent chemotherapy and subsequently developed DM, the death rate was 80% with a median survival of nine months after DM.
Figure 1. Cumulative incidence of any (A) and site-specific first distant metastases (DMs) among all patients compared to incidence death without DM failure (B) from time of treatment completion.
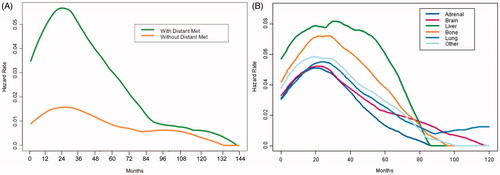
Due to loss of follow-up and death, reliable salvage treatment data was available for 314 (69%) patients, of which 294 received additional treatment. With regards to treatment 194 (62%), 171 (54%), and 58 (18%) patients received radiation, chemotherapy, and/or surgery a component of salvage treatment. To visualize variations in the rate of death over time, the hazard of death was plotted over time stratified by first DM (). Among patients who did not develop DM, the hazard of death gradually rose to a relatively low peak 2–3 years after RT followed by a gradual decline. In contrast, among patients who developed DM the hazard of death spiked to a higher peak at approximately the same time (2–3 years after RT) followed by a more rapid fall. At approximately 7–8 years after RT the risk of death among patients who developed DM was roughly similar to those who did not (). Stratification by different sites of first DM revealed similar hazard function behavior. Of note, patients who develop first DM within the bone or liver exhibited generally higher hazards of death than those who developed first DM elsewhere (). The behavior of these hazard functions prompted survival analysis truncation at five years after RT to comply with the proportional hazards assumption required of Cox regressions.
Figure 2. Kernel-based estimate of the hazard of death over time among patients who develop distant metastatic (DM) disease compared with those who do not (A) and comparing between different first sit of DM disease (B). X-axis (time) is from time of treatment completion. Data were treated as left truncated at time of first DM.
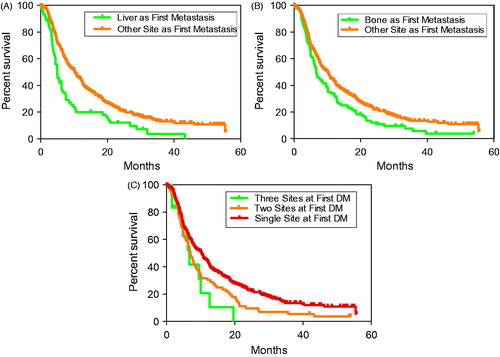
To more quantitatively assess which DM sites were associated with worse survival, survival analyzes were utilized to compare the 456 patients who developed DM. To prevent lead-time bias, survival analysis was initiated at time of first DM. Similar to the previous analysis, patients who developed first DM within the liver exhibited worse survival (median: 5.0 months after DM) compared to patients who developed DM elsewhere (HR = 1.8, 95% CI 1.3–2.4, p < 0.001) (). Development of first DM within bone exhibited the second worst rate of survival (median: 6.7 months; HR = 1.4, 95% CI 1.1–1.8, p = 0.01) (). Comparing survival following first DM within the lung (median: 10.2 months), brain (median: 10.3 months), adrenal (median: 10.0 months), or other sites (median: 7.4 months) did not reveal significant differences with all patients exhibiting DM (all p > 0.05).
Figure 3. Overall survival in patients who developed distant metastatic disease. Survival time initiated from time of metastatic disease diagnosis. Specific curves compare patients who do or do not develop liver (A) and bone (B) as sites of first metastases and among patients who developed single and two or three separate sites of first metastases (C).
Multivariate analysis adjusting for age at diagnosis, tumor stage, histology, time between radiation completion and DM diagnosis, and performance status confirmed first metastasis within the liver (HR = 1.9, 95% CI 1.4–2.5, p < 0.001) or bone (HR = 1.3, 95% CI 1.0–1.7, p = 0.04) to exhibit significantly worse OS. For comparison, multivariate Fine and Gray regression was conducted to associate disease and treatment factors with death without DM. This analysis identified high KPS (80–100 vs. <80: HR = 1.60, 95% CI 1.25–2.04, p = 0.002) and 3D-CRT (3D-CRT vs. PBT: HR = 1.82, 95% CI 1.22–2.73, p = 0.004) to be significantly associated with increased risk of death without DM. In contrast, non-squamous histology (HR = 0.56, 95% CI 0.46–0.70, p < 0.001), concurrent chemotherapy (HR = 0.67, 95% CI 0.52–0.87, p < 0.001), and twice daily (BID) fractionation (HR = 0.55, 95% CI 0.32–0.97, p = 0.04) were associated with decreased risk of death without DM (Supplementary Table 2, available online at http://www.informahealthcare.com).
Table 2. Univariate analyses associating treatment and demographic factors with any and organ-specific distant metastases.
Multiple first DM sites
At diagnosis of first DM, 78 patients (17%) exhibited disease at two simultaneous sites, while 12 (3%) exhibited disease at three sites. No patients exhibited first DM at more than three sites. Patients with DM limited to a single site exhibited the best survival (median: 10.3), compared with patients with involvement of two (median: 7.0 months) or three (median survival: 6.7 months) sites (HR = 1.4, 95% CI 1.1–1.7, p = 0.001) (). Significance was maintained on multivariate analysis (HR = 1.3, 95% CI 1.1–1.7, p = 0.007). The two sites associated with the highest frequency of multiple simultaneous metastases were liver (51%) and bone (41%). Brain metastasis was associated with the lowest frequency (24%). Interestingly, on multivariate analysis adjusting for multiple DM sites produced a non-significant association between first DM within the bone and survival (HR = 1.2, 95% CI 0.9–1.5, p = 0.19). However, following this adjustment the significant association with first DM within the liver was maintained (HR = 1.7, 95% CI 1.3–2.4, p < 0.001).
Predictors of unfavorable first distant metastatic site
Multivariate analysis identified non-squamous histology (HR = 1.66, 95% CI 1.36–2.03, p < 0.01), BID RT fractionation (HR = 1.69, 95% CI 1.13–2.54, p = 0.01), and advanced AJCC Stage (stage III vs. II/I: HR = 2.08, 95% CI 1.50–2.87, p < 0.001) to be associated with the development of any DM (). To illustrate the effects of histology and stage, stratified cumulative incidence plots are shown in .
Figure 4. Cumulative incidence of developing distant metastatic (DM) disease and site-specific DM. Cumulative incidence curves display incidence of any DM stratified by histology (A) and overall disease stage (B). Incidence of first DM within the liver is shown stratified by histology (C) and incidence of first DM within the bone stratified is shown stratified by time between diagnosis and RT initiation (D).
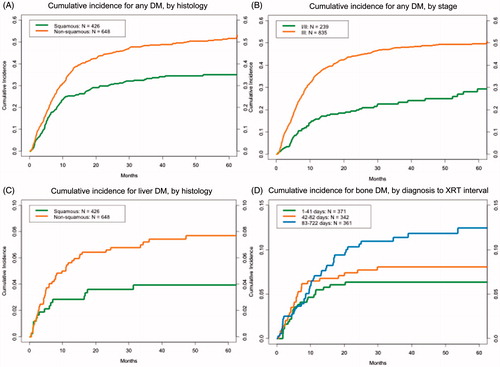
Multivariate Fine-Gray regression was conducted to determine predictors of first DM in the two prognostically unfavorable sites: liver and bone. To avoid double counting, seven patients who developed first DM simultaneously in the bone and liver were only considered in the liver DM analysis. Patients developed DM within the liver at a median of 4.9 months after RT completion (range: 0.5–47.2 months). Only non-squamous histology was significantly associated with first DM within the liver (HR = 2.04, 95% CI 1.16–3.60, p = 0.01) (). However, patients developed DM within the bone at a median of 6.9 months (range: 0.3–53.7 months). Time to development of first DM within the bone compared with liver was not significantly different. Delay between diagnosis and RT administration (third vs. first tertile: HR = 2.3, 95% CI 1.26–4.21, p = 0.007) in addition to advanced stage (stage III vs. II/I: HR = 2.37, 95% CI 1.11–5.06, p = 0.03) were associated with development of first DM within bone (, ).
Discussion
Our major findings were as follows. First, the sites of first DM associated with worse survival were liver and bone metastasis in addition to multiple metastatic sites at first DM. Interestingly, adjustment for multiple first DM sites maintained the association between liver as first DM and worse OS, while the association between bone as first DM and survival became non-significant. Factors predictive of any DM were non-squamous histology, advanced initial AJCC stage, and BID RT fractionation, BID RT fractionation represents a specific RT regime at our institution: 1.2 Gy BID to a total dose of 69.6 Gy. It is the practice of our institution to use BID fractionation when single fraction dose constraints cannot be met. As such the association between BID treatment and higher frequency of DM may reflect selection bias rather than adverse effects of BID treatment. Finally, factors predictive of first DM within prognostically unfavorable sites were non-squamous histology for liver and a delay between diagnoses and RT and advanced initial stage for bone ().
Our observed frequency of DM failure (42%) is consistent with that presented in other studies assessing treated with definitive radiation (ranging 33–59%) [Citation1–3] and higher than those assessing definitive surgical management (range 23–42%) [Citation16–18]. Predictors of DM after definitive treatment for NSCLC have been elucidated in a number of reports. Cox et al. identified non-squamous histology to be associated with DM from a pooled analysis of four RTOG trials totaling 1765 patients [Citation2]. These results were echoed by a more recent analysis by Hung et al. who also identified advanced T-stage and non-squamous histology to be associated with DM in a Taiwanese cohort of surgically resected patients with early stage NSCLC [Citation19]. Consistent with prior studies, our current analysis also identified non-squamous histology and advanced initial AJCC stage to be associated with DM (). A novel finding of our current study is that patients with metastasis ≥5 years after RT completion exhibited similar hazards of death compared with patients who did not develop metastasis. Disease in these patients may represent a more indolent metastatic phenotype or exhibit targetable markers. Regardless of the reason, we felt that Cox regression analysis utilizing survival data five years after RT should not be conducted in this cohort due to violation of the proportional hazards assumption. Whether this phenomenon extends to other datasets requires validation.
It is difficult to determine the mechanisms responsible for metastatic dissemination and worse prognosis associated with first metastases. As this analysis revealed different factors to predict first DM within the liver versus bone, we hypothesize different dissemination mechanisms. One explanation is that tumor subclones within locoregional disease require more time to develop metastatic potential to the bone. This is supported by the finding that >82 day delay in local treatment and advanced AJCC stage were predictive of first DM within bone. However, the molecular machinery required for first DM within the liver may reflect more intrinsic tumor biology and may not require the same extent of temporal evolution. The finding that only histology significantly predicted for first DM within the liver supports this hypothesis.
In addition, the association between first DM within the liver or bone with worse survival may also reflect different mechanisms. We hypothesize that these metastases could affect survival due to their location at a site that compromises critical organ function or because they reflect aggressive underlying cancer biology, serving as a sign of more widely disseminated disease. The latter of the two mechanisms may explain the association between bone as first DM and worse survival. This hypothesis is supported by the finding that the association between bone as first metastasis and survival becomes non-significant after controlling for multiple sites of metastatic involvement. In contrast, liver metastases may act through the other mechanism, resulting in death by compromising critical liver functions. Such a hypothesis may explain the survival advantage conferred by aggressive early liver metastases treatment in other disease sites including colorectal cancers [Citation20,Citation21]. In contrast, brain metastases were associated with lowest number of concomitant first metastases in addition to the longest median survival (10.3 months from DM). As such, solitary brain metastases may represent metastatic biology with more limited potential and therefore warrant aggressive treatment, as suggested by a past analysis from our group [Citation9]. A recent analysis by our group provides further support for the treatment of patients with a limited burden of metastatic disease [Citation12]. To further investigate these hypotheses, we are currently investigating the sequential development of DM sites after first DM within this study population. These findings largely parallel a report by O’Connell et al. who assessed the prognostic implication of organ involvement with metastasis at start of treatment. This group identified a strong association between bone metastasis and survival, a borderline association between liver metastasis and survival, and a non-significant association between brain metastases and survival [Citation22].
Various limitations of this analysis deserve mention. First, not all patients received comprehensive whole body imaging during follow-up. Instead, routine follow-up practices largely reflected current practice guidelines, which utilize chest CT with imaging extending down to the liver and adrenal glands [Citation23]. However, imaging follow-up was practitioner dependent with noted heterogeneity especially in the surveillance of sites, such as the central nervous system. Second, there is noted heterogeneity in patient demographics and received treatment. Furthermore, AJCC lung cancer staging underwent two changes during the study period, transition from 5th to 6th edition in 2002 and then to 7th edition in 2010 [Citation24]. This change did have implications in the classification of metastatic disease. In addition, generally there was observed to be a long delay between diagnosis and treatment time. This finding reflects not only the substantial utilization of induction chemotherapy but also the patient population treated this tertiary center, who often are diagnosed at a home center and often seek a second (or multiple additional) opinions before starting treatment. Also, due to the limited number of patients exhibiting first metastasis within the liver and bone, this analysis exhibited limited power to detect less significant factors predictive of site-specific metastases. Given the complexity of treatment options after DM, we were also unable to account for treatments given after metastatic disease diagnosis, which may likely affects survival prognosis. Despite these limitations, notable strengths deserve mention. To our knowledge, this is one of the first analyses that investigate specific sites of NSCLC metastases after definitive radiation in a modern cohort. In addition, statistical methods were utilized throughout this study that took into account competing risks.
In conclusion, we present evidence that first DM in either the liver or bone after definitive RT in NSCLC is associated with worse prognosis. Further analysis identified distinct factors predictive for DM within each site. Based on these data, we hypothesize that different mechanisms are responsible for the metastatic dissemination and subsequent survival prognoses. Given the current interest in the study and aggressive treatment of metastatic disease, we believe that this clinical data generates interesting hypotheses into the behavior and underlying heterogeneity of metastatic disease in NSCLC and hint at underlying differences in molecular phenotype. We are currently pursuing the analysis of biologic and molecular markers to provide molecular biology context for this relationship. Ultimately, given the retrospective nature, results of this study must be viewed as hypothesis generating and require validation in the prospective setting.
Supplementary_Table_2.docx
Download MS Word (55.7 KB)Supplementary_Table_1.docx
Download MS Word (12 KB)Acknowledgments
This work was supported in part by Cancer Center Support (Core) Grant CA016772 to The University of Texas MD Anderson Cancer Center. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Komaki R, Scott CB, Byhardt R, Emami B, Asbell SO, Russell AH, et al. Failure patterns by prognostic group determined by recursive partitioning analysis (RPA) of 1547 patients on four radiation therapy oncology group (RTOG) studies in inoperable nonsmall-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1998;42:263–7.
- Cox JD, Scott CB, Byhardt RW, Emami B, Russell AH, Fu KK, et al. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys 1999;43:505–9.
- Komaki R, Allen PK, Wei X, Blumenschein GR, Tang X, Lee JJ, et al. Adding Erlotinib to Chemoradiation Improves Overall Survival but Not Progression-Free Survival in Stage III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2015;92:317–24.
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nature Rev Clin Oncol 2011;8:378–82.
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009;9:239–52.
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastas Rev 1989;8:98–101.
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer 2006;106:1624–33.
- Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol 2001;19:1344–9.
- Hu C, Chang EL, Hassenbusch SJ, 3rd, Allen PK, Woo SY, Mahajan A, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998–2004.
- Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012;118:2962–70.
- Parlak C, Mertsoylu H, Guler OC, Onal C, Topkan E. Definitive chemoradiation therapy following surgical resection or radiosurgery plus whole-brain radiation therapy in non-small cell lung cancer patients with synchronous solitary brain metastasis: a curative approach. Int J Radiat Oncol Biol Phys 2014;88:885–91.
- Sheu T, Heymach JV, Swisher SG, Rao G, Weinberg JS, Mehran R, et al. Propensity Score-Matched Analysis of Comprehensive Local Therapy for Oligometastatic Non-Small Cell Lung Cancer That Did Not Progress After Front-Line Chemotherapy. Int J Radiat Oncol Biol Phys 2014;90:850–7.
- Hess KR, Levin VA. Getting more out of survival data by using the hazard function. Clin Cancer Res 2014;20:1404–9.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509.
- Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54.
- Demicheli R, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol 2012;7:723–30.
- Varlotto JM, Yao AN, DeCamp MM, Ramakrishna S, Recht A, Flickinger J, et al. Nodal stage of surgically resected non-small cell lung cancer and its effect on recurrence patterns and overall survival. Int J Radiat Oncol Biol Phys 2015;91:765–73.
- Varlotto JM, Medford-Davis LN, Recht A, Flickinger JC, Schaefer E, DeCamp MM. Failure rates and patterns of recurrence in patients with resected N1 non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:353–9.
- Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang BS, Wu YC. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol 2012;7:1115–23.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol2007;25:4575–80.
- Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77:1254–62.
- O'Connell JP, Kris MG, Gralla RJ, Groshen S, Trust A, Fiore JJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol 1986;4:1604–14.
- Rubins J, Unger M, Colice GL, American College Of Chest P. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest 2007;132:355S–67S.
- Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E. Jr., The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol 2012;4:128–34.
