Abstract
Background: The aim of the study was to calculate the rate of chemotherapy-induced amenorrhea (CIA) after treatment with different adjuvant therapies in patients with breast cancer and to evaluate the risk factors for CIA based on the quality of evidence.
Patient and methods: A search of PubMed and ISI Web of Science was performed. All published trials with female breast cancer patients who received adjuvant chemotherapy and presented data on the rate of CIA were considered eligible. The pooled rates of CIA were calculated by random effects model. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each potential risk factor for CIA by using the generic inverse weighted method.
Results: We identified 580 potentially relevant studies, of which 75 were included in the analysis. Among 75 eligible studies, 19 different definitions of CIA have been used. The pooled rate of CIA was 55% (95% CI 50–60%) including 23 673 patients from 74 studies. The rate of CIA was increased by age with an estimate of 26% (95% CI 12–43%), 39% (95% CI 31–58%), and 77% (95% CI 71–83%) for women <35, 35–40, and >40 years old, respectively. Two risk factors were associated with the occurrence of CIA and were supported by strong level of evidence: older age (>40 years old), and the use of tamoxifen.
Conclusions: This meta-analysis summarized the updated evidence on the impact of different adjuvant treatment regimens for breast cancer in menstruation and could serve as a helpful guide for oncologists during the discussion with their patients on fertility issues before decision on adjuvant therapy is made. A uniform definition of CIA is essential in future studies to make the interpretation of results more reliable.
The incidence of breast cancer in young women has been relative stable around the world during the last 20 years [Citation1]. However, breast cancer in young women remains a challenge for the oncologists: the high risk for recurrence in this patient population [Citation2] has led to more aggressive therapeutic strategies including adjuvant chemotherapy that improves the survival rates [Citation3] but, at the same time, chemotherapy negatively influences patients’ quality of life [Citation4].
One of the main issues that influence patients’ quality of life after adjuvant chemotherapy is the risk for infertility [Citation4]. Chemotherapy-induced amenorrhea (CIA) is a well known toxicity after chemotherapy in young breast cancer patients that has traditionally served as a surrogate marker of infertility. The majority of young breast cancer patients have concerns about treatment-induced infertility [Citation5,Citation6] and in some cases these concerns influence their treatment decision [Citation5–8]. However, the discussion about CIA and infertility risk between oncologists and their patients remains limited [Citation5,Citation6] and a potential barrier is the lack of knowledge and consistent evidence on these issues [Citation9,Citation10].
The link between adjuvant chemotherapy and amenorrhea has been well established, but the specific effect of individual chemotherapeutic agents and regimens on risk for amenorrhea have not been well characterized. Indeed, the incidence of CIA among eligible studies ranges between 10% and 93% [Citation11]. The wide range of CIA rates reflects the problems occurred in the current literature mainly due to the variations in the definition of CIA, the differences in follow-up periods, and the characteristics of the included patients among different studies. Based on the current literature, risk tools have been created to help clinicians and patients in decision making [Citation12]. However, due to the heterogeneity of existing studies the ranges of CIA for each chemotherapy regimen on these tools are very wide and thus the interpretation of the results is challenging.
Recently, a meta-analysis tried to deal with some of these challenges [Citation13]. However, the authors did not take into account the complexity of confounding factors in analyses. In addition, there was no quantitative evaluation of the risk for CIA in regard to different chemotherapeutic agents. Finally, the quality of evidence was not assessed, thus making the interpretation of results from clinicians difficult.
A pooled estimate, based on the quality of the current literature, of risk for CIA regarding patient- and treatment characteristics could help clinicians to discuss with their young breast cancer patients about the treatment plan and the need for fertility preservation options.
We, therefore, performed this meta-analysis to provide the most up-to-date estimate of the risk of CIA after breast cancer treatment with chemotherapy. Our secondary aim was to identify potential risk factors for CIA obtained from multivariate analyses and grade the factors based on the quality of evidence.
Methods
Searching strategy
This meta-analysis was conducted in accordance with the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [Citation14].
We searched two databases (PubMed and ISI Web of Science) to identify potentially eligible studies for the systematic review without year or language restriction. The following algorithm was used: (amenorrhea OR premature menopause OR early menopause OR ovarian failure) AND (adjuvant OR postoperative OR prophylactic) AND (chemotherapy OR cytotoxic) AND (breast OR mammary) AND (cancer OR carcinoma OR malign* OR tumor). The last searching was updated on April 2015.
To ensure that all relevant studies were included, we reviewed the references of selected review articles on the topic and we also conducted secondary referencing by manually reviewing reference lists of potentially eligible articles.
In studies with multiple publications from the same cohort of patients, only data from the most recent publication were included in the systematic review and in meta-analysis.
Study selection
Studies were included in our systematic review if they fulfilled all the following criteria: 1) studies of female breast cancer patients that received adjuvant (postoperative) chemotherapy; 2) published studies that presented data on rate of amenorrhea after chemotherapy (irrespective of definition of amenorrhea).
For the meta-analysis of risk factors, we included only studies that identified risk factors obtained from multivariable analyses that were adjusted for at least one potential confounder.
We excluded studies that included only patients with hereditary breast cancer, studies with only metastatic breast cancer, studies that used neoadjuvant chemotherapy, studies with few patients (n < 10), studies that did not include adequate data to calculate rate of amenorrhea (no contact with primary authors was planned), and studies that used GnRH-analogs during chemotherapy. In case of randomized trials that investigated the role of GnRH-analogs in preserving amenorrhea during chemotherapy, we included only patients from standard arms, namely patients that received chemotherapy without the addition of GnRH-analogs.
Data extraction
One investigator selected articles that potentially met our inclusion criteria on the basis of their titles and abstracts. For the eligible studies, two investigators abstracted the data independently on a predefined form. In case of discrepancies, the two investigators discussed and resolved the differences.
The following data were collected from each study: first author’s last name, year of publication, country of origin, type of study, duration of follow-up, definition of amenorrhea, total number of patients, patients with amenorrhea based on study’s definition, age of included patients, type of chemotherapy used, duration of chemotherapy, dose intensity, use of tamoxifen, risk factors for CIA with adjustment for at least one potential confounder in multivariate analysis.
Quality assessment
Two investigators independently assessed each eligible study for methodological quality using a 14-item checklist that selected from a bank of items for evaluating the risk of bias of observational studies [Citation15] (Supplementary Table S1, available online at http://www.informahealthcare.com).
Each item of a selected study was assigned one point, with the highest possible score for any one study being 14. Studies scoring more than 9 points (>70% or more of the maximum attainable score) were rated as high quality, studies scoring 7–9 points were rated as moderate quality, and studies scoring 6 or fewer points (lower than 50% of maximum attainable score) were regarded as low quality.
The quality of eligible studies was then used to categorize the risk factors into four levels of evidence, i.e. strong, moderate, weak, or inconclusive. Besides the study quality, we used the consistency of findings, i.e. the degree of similarity in the effect sizes of the different studies, to categorize each risk factor.
Level of evidence for risk factors was defined as follows: Strong, in case of consistent findings including at least two high-quality studies; moderate, in case of consistent findings including one high-quality study; weak, in case of inconsistent result or consistent results without a high-quality study; inconclusive, if there is only one eligible study. Similar adaptations have been previously used in the meta-analysis of risk factors in breast cancer [Citation16].
Data synthesis and analysis
The main outcome of interest for this analysis was the pooled overall rate (%) of CIA after adjuvant chemotherapy for breast cancer; exact binomial 95% CIs were subsequently calculated. When different definitions of CIA were calculated in the same study, we used the CIA rate observed at 12 months after chemotherapy.
We used a random effects model to produce a pooled overall rate for CIA and we then calculated the CIA in different subgroups of interest: type of studies; age and type of chemotherapy; definition of CIA used; tamoxifen use; dose intensity; duration of chemotherapy. We proceeded to the calculation of pooled CIA rates and their 95% CI if there were at least two studies with more than 100 patients in each subgroup.
For each risk factor, we abstracted odds ratios (ORs), hazard ratios (HRs), or relative risks (RRs) and associated 95% CIs, based only on factors obtained from multivariable analyses that were adjusted for at least one potential confounder. As a result of the high risk for CIA, the discrepancies between the ORs and HRs/RRs are substantial [Citation17]. Considering the fact that most of the studies that investigated risk factors calculated ORs (14 of 17 studies), we decided to exclude the three studies that calculated HRs.
We pooled the ORs from all studies that provided data adjusted at least one potential confounder by using the generic inverse weighted method, i.e. studies were weighted by the inverse of the standard error of the log transformed ORs. We calculated the standard errors of these log ORs using published confidence intervals (CIs) and then used these to weight the studies according to the precision of the OR.
Unadjusted effect estimates were used as alternatives if studies did not observe an association on bivariate comparison and did not therefore include a risk factor of interest on the multivariate model.
We assessed the presence of statistical heterogeneity among the studies using the Q statistics and the magnitude of heterogeneity using the I2 statistic. We considered a p-value ≤0.10 or an I2 value of greater than 50% as indicative of substantial heterogeneity.
The presence of publication bias was evaluated qualitatively using a funnel plot. All reported p-values are two-sided, with significance set at p ≤ 0.05. All statistical analyses were performed with RevMan 5.3 (Review Manager, Version 5.3; The Cochrane Collaboration, 2014) and StatsDirect (StatsDirect Ltd. UK, 2013).
Results
Eligible studies
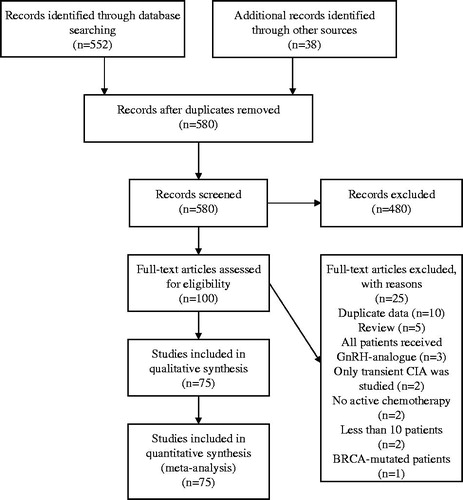
We identified 580 potentially relevant studies, of which 75 were included in our analysis (; online-only reference list). Thirty-one of the 75 (41%) studies were randomized trials, 23 (31%) were retrospective cohort studies, and 15 (20%) were prospective cohort studies (Supplementary Table S2, available online at http://www.informahealthcare.com).
Pooled rate of CIA
The pooled rate of CIA was 55% (95% CI 50–60%) including 23 673 patients from 74 studies (instead of 75 eligible studies because one study presented data only after multivariate analysis without data to calculate rate of amenorrhea). The pooled rate of CIA ranged between 45% and 61% according to the type of study with the randomized trials giving the higher estimate and the retrospective cohort studies the lowest ().
Table 1. Pooled rates of chemotherapy-induced amenorrhea.
The rate of CIA was increased by age with an estimate of 26% (95% CI 12–43%), 39% (95% CI 31–58%), and 77% (95% CI 71–83%) for women <35, 35–40, and >40 years old, respectively.
The risk for CIA according to adjuvant chemotherapy regimen and age is presented on . Women ≥40 years old treated with either CMF or anthracycline-based chemotherapy other than AC ×4 had the highest risk for CIA (>70%). The low risk group for CIA (<30%) included women <35 years old irrespective of chemotherapy as well as women <40 years old that were treated with either anthracycline- and taxane-based chemotherapy or with AC ×4 (). Due to lack of adequate data (<100 patients), we did not calculate the pooled rate of CIA in patients treated with only taxane-based chemotherapy. A flowchart to illustrate the risk classification of CIA based on age and type of chemotherapy is shown on .
Figure 2. Flowchart of risk for chemotherapy-induced amenorrhea based on age and type of chemotherapy. AC: doxorubicin-cyclophosphamide.
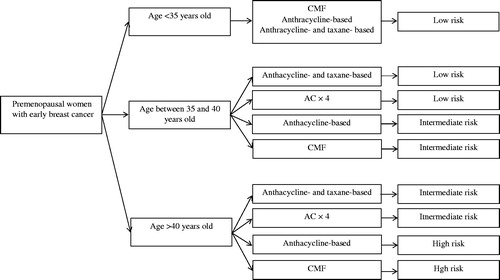
Table 2. Risk for chemotherapy-induced amenorrhea based on chemotherapy type and age.
Risk factors for CIA
Of the 14 studies (including 3661 patients) that investigated risk factors by using ORs as estimates, seven were high, six were moderate, and one was low quality ().
Table 3. Summary of risk factors from multivariate analyses for chemotherapy-induced amenorrhea in breast cancer patients.
The risk factors that were associated with the occurrence of CIA and were supported by strong level of evidence were: older age (>40 years old or age as continuous variable), and the use of tamoxifen. Two additional variables were not associated with increased risk for CIA with a strong level of evidence: body mass index (BMI) and the age at menarche.
The use of taxanes was not found to be associated with higher risk for CIA; however, the level of evidence was weak. We found inconclusive evidence to other potential risk factors: smoking, educational level, nulliparity, trastuzumab use, dose intensity, and duration of chemotherapy.
Three studies identified risk factors for CIA by using HR as estimates. A separate meta-analysis of these three studies revealed the same risk factors for CIA (age and tamoxifen use). No other risk factor could be identified as significant in the meta-analysis (data not shown).
Definition of CIA
Among 75 eligible studies, 19 different definitions have been used (Supplementary Table S3, available online at http://www.informahealthcare.com). The definitions could be differed on the interval from chemotherapy to amenorrhea (during chemotherapy; at the start of chemotherapy; at the end of chemotherapy; within 3–12 months from chemotherapy) and the duration of amenorrhea (from 3 months to permanent amenorrhea).
The most commonly used definition (no menstrual bleeding within at least 12 months from start of chemotherapy) was used in nine (12%) studies. Seven (9%) studies used different durations of amenorrhea and presented results on different definitions. Three (4%) studies used a laboratory criterion in the definition of amenorrhea (rise of gonadotropins) whereas five (7%) used the criterion of non-menstrual recovery to identify patients with permanent amenorrhea. Fifteen (20%) studies presented data on CIA without a clear definition of CIA.
The rates of CIA in eligible studies ranged between 38% and 70% according to different definitions of CIA with the higher rate for studies that used a short duration of amenorrhea (three months) in their definition and the lower rate for studies that used a more strict definition (amenorrhea at least six months within 12 months from chemotherapy).
Discussion
Our meta-analysis provides updated insights into the risk for CIA in women with breast cancer and intends to serve as a tool to help oncologists in counseling patients on fertility issues. Through an extensive systematic review, we summarized updated evidence on the impact of different adjuvant treatment regimens for breast cancer in menstruation. We also investigated the impact of several risk factors on CIA and categorized the risk factors according to the level of evidence. Age and tamoxifen use were found to be the most important factors associated with higher risk for CIA with a strong level of evidence. The use of taxanes in addition to anthracycline-based chemotherapy was not associated with higher risk for CIA but the level of evidence for this observation was weak. However, the heterogeneity of included studies is high mainly due to the lack of uniform definition of CIA.
Many young patients with breast cancer have concerns on fertility when discussing with their oncologist about adjuvant therapy; these concerns may influence their treatment decisions [Citation5–7]. Taking into account the effect of fertility issues on patients’ quality of life, the American Society of Clinical Oncology recommends that oncologists discuss the risk of infertility and fertility preservation options in patients with cancer as early as possible before treatment starts [Citation18]. It is, therefore, essential for oncologists to have access to the current level of evidence on the topic and inform their patients knowing the limitations of the existing literature.
The most important drawback on the current literature is the lack of uniform definition of CIA. In the mid-1990s, a systematic review by Bines et al. underscored the need for uniform definition of CIA and proposed one based on the duration of amenorrhea (≥6 months) [Citation19]. A similar definition has been adopted from the American College of Obstetricians and Gynecologists [Citation20]. However, we found 19 different definitions for CIA in the eligible studies. The impact of this wide variation of definitions for these patients may be greater than we believe. In fact, the applicability and generalization of the results from different studies that used various definitions for the main outcome is problematic. How can we inform our patients about the infertility risk of a proposed treatment if the evidence is not based on a uniform definition? Our meta-analysis of pooled rates from different CIA definitions further outlined the problem as we found a difference of 32% between the lowest and highest CIA rate according to definition used. Our meta-analysis could also be considered as prone to bias due to the diversity of definitions that were used from the eligible studies. However, we tried to overcome this obstacle by choosing for our analyses the most commonly used definition (amenorrhea ≥12 months from chemotherapy) when different definitions of CIA were calculated in the same study.
Based on the current systematic review and the evidence derived from studies with long-term follow-up, where resumption of menses occurs often within two years of CIA [Citation21,Citation22], we propose that the definition of CIA should be based on the presence of amenorrhea for at least two years commencing within two years of chemotherapy with no resumption of menses during this period. One could argue that this definition is also problematic since there are some patients that resume menses even 2–3 years after CIA [Citation22–24]; however, we assume that the two-year cutoff is an acceptable compromise between the necessity for a uniform and reliable definition and the resources that a prospective study investigating the risk for CIA in cancer patients is needed.
Another aspect that should be kept in mind when interpreting the results of the studies on CIA is the value of the term CIA per se. Irrespective of the definition used for CIA, cessation of menses is only a surrogate marker for ovarian function and should not be considered synonymous to true ovarian failure. In fact, it has been found that estrogen levels can remain high despite the presence of CIA for more than 12 months [Citation24,Citation25]. Conversely, recovery of menstruation after chemotherapy does not rule out follicular depletion and fertility cannot be guaranteed [Citation26,Citation27]. These observations suggest that more accurate indicators of ovarian function are needed to properly inform cancer patients about their risk for infertility due to cancer treatment. Among several markers that have been studied, anti-Müllerian hormone (AMH) seems to be the most promising one. Indeed, serum AMH has been associated with recovery of ovarian function in young women during and after chemotherapy [Citation28]. Several prospective studies are ongoing to determine the role of AMH as marker of the preservation of ovarian reserve [Citation29,Citation30] and more important its role as predictor of pregnancy rates in cancer survivors.
In addition to the limitations because of the diversity of CIA definitions and the clinical value of CIA per se, there are some methodological drawbacks of the meta-analysis that need attention. First, this is a study level and not an individual patient meta-analysis (IPM) which is considered the “gold standard” of the meta-analysis and has the advantage to minimize the heterogeneity due to the ability to define exposures and outcomes consistently across studies [Citation31]. However, the large number of primary studies and the presence of a considerable number of eligible studies that published more than 20 years ago make the effort to perform IPM impossible. Second, there were several sources of heterogeneity among eligible studies included the definition of CIA, the duration of follow-up, the number of included patients and events. We tried to limit this potential source of bias by choosing random effects models for meta-analyzes of rates. Publication bias and selective reporting are also potential limitations but are difficult to assess.
Despite these drawbacks, our meta-analysis offers valuable information to the oncologists. The results that are summarized in could replace the tables that are commonly used to inform breast cancer patients about their risk for amenorrhea resulting from adjuvant breast cancer chemotherapy as it includes the most current evidence. Regarding the risk factors for CIA we found that age is the strongest risk factor (six-fold increased risk for CIA for patients ≥40 years old) whereas tamoxifen use is associated with nearly two-fold increased risk for CIA. On the contrary, age at menarche and BMI do not influence the risk for CIA. These observations are supported by high level of evidence. The addition of taxanes does not seem to influence the risk for CIA; however, the level of evidence is weak due to the inconsistency of results. These risk factors alone do not accurately predict who will develop CIA or not but they can be used to inform breast cancer patients on the magnitude of risk for amenorrhea after adjuvant chemotherapy.
From our systematic review and meta-analysis, it is clear that future studies evaluating the risk for CIA in breast cancer patients should clearly define the term CIA with a uniform definition as the one we propose above. Furthermore, several gaps in the literature have been outlined by our meta-analysis; these gaps need to be addressed by future studies. For instance, several potential risk factors for CIA are not extensively studied and the evidence is inconclusive. Two of those factors are of great interest: the dose intensity and the trastuzumab use. Dose dense chemotherapy, namely the delivery of higher amount of drug per unit of time, as adjuvant treatment strategy in breast cancer patients has been associated with better overall survival compared to conventional chemotherapy and is considered the preferred treatment option for patients with high risk breast cancer [Citation32]. Theoretically, dose dense strategies should lead to higher risk for CIA. Our meta-analysis of pooled rates for CIA revealed considerably high rates for patients received dose dense chemotherapy. However, only one study included dose density as a variable in multivariate analysis and found no negative effect of dose dense chemotherapy in CIA. Thus, the evidence is inconclusive and further studies are warranted. In the same way, the effect of trastuzumab in the risk for CIA has only been investigated by one study that could not find any association. Until further studies are available, the question on the potential effect of trastuzumab on fertility is still open.
In conclusion, this meta-analysis could serve as a helpful guide for oncologists during the discussion with their patients on fertility issues before decision on adjuvant therapy is made. The discussion should include the inherent risk for amenorrhea of each chemotherapy combination, the potential role of risk factors (including age, tamoxifen use) on this risk, the fertility preservation options, and the psychosocial aspects of the decision. Further research is needed to improve our ability to predict the ovarian function after chemotherapy, and to understand the role of other potential risk factors for amenorrhea, especially those related to newer targeted therapies (i.e. trastuzumab) or treatment strategies that could positively influence prognosis (i.e. dose dense chemotherapy).
SONC-2016-0002.R1_-_Supplementary_material_Valachis.docx
Download MS Word (40.2 KB)Disclosure statement
The authors have no conflict of interest to declare.
References
- Narod SA. Breast cancer in young women. Nat Rev Clin Oncol 2012;9:460–70.
- Azim HA, Jr, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 2012;18:1341–51.
- Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44.
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:386–405.
- Ruddy KJ, Gelber SI, Tamimi RM, Ginsburg ES, Schapira L, Come SE, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol 2014;32:1151–6.
- Partridge AH, Gelber S, Peppercorn J, Ginsburg E, Sampson E, Rosenberg R, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 2004;22:4174–83.
- Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst 2015;107:djv202.
- King JW, Davies MC, Roche N, Abraham JM, Jones AL. Fertility preservation in women undergoing treatment for breast cancer in the UK: a questionnaire study. Oncologist 2012;17:910–16.
- Adams E, Hill E, Watson E. Fertility preservation in cancer survivors: a national survey of oncologists' current knowledge, practice and attitudes. Br J Cancer 2013;108:1602–15.
- Duffy C, Allen SM, Dube C, Dickersin K. Oncologists' confidence in knowledge of fertility issues for young women with cancer. J Cancer Educ 2012;27:369–76.
- Torino F, Barnabei A, De Vecchis L, Sini V, Schittulli F, Marchetti P, et al. Chemotherapy-induced ovarian toxicity in patients affected by endocrine-responsive early breast cancer. Crit Rev Oncol Hematol 2014;89:27–42.
- Available from: http://www.livestrong.org/we-can-help/fertility-services/risks/. [Accessed March 5, 2016].
- Zhao J, Liu J, Chen K, Li S, Wang Y, Yang Y, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat 2014;145:113–28.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group JAMA 2000;283:2008–2012.
- Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 2012;65:163–78.
- Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat 2014;144:443–55.
- Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ 1998;316:989–991.
- Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013; 31:2500–10.
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 1996;14:1718–29.
- Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinology and Infertility. 6th ed. Lippincott Williams & Wilkins: Philadelphia, PA, 1999.
- Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer 2010;116:3102–111.
- Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat 2009;115:335–42.
- Hargis JB, Nakajima ST. Resumption of menses with initiation of letrozole after five years of amenorrhea on tamoxifen: caution needed when using tamoxifen followed by aromatase inhibitors. Cancer Invest 2006;24:174–7.
- Smith IE, Dowsett M, Yap YS, Walsh G, Lønning PE, Santen RJ, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol 2006;24:2444–7.
- Amir E, Seruga B, Freedman O, Clemons M. Amenorrhoea, menopause, and endocrine therapy for breast cancer BMJ. 2009;339: b4261.
- Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: Preliminary results. Reprod Biomed Online 2010;20:280–85.
- Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Müllerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer 2013; 49:3404–3411.
- Peigné M, Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: a systematic review. Reprod Biol Endocrinol 2014;12:12–26.
- Memorial Sloan Kettering Cancer Center. Serum Biomarkers to Characterize the Effects of Therapy on Ovarian Reserve in Premenopausal Women With Early-stage Breast Cancer or BRCA Mutations. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT00823654. NLM Identifier: NCT00823654. [Accessed September 27, 2015].
- Peking University People's Hospital. Study of Preservation of Ovarian Reserve Function During Chemotherapy for Breast Cancer Patients at Reproductive Age (PORF). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT02430103. NLM Identifier: NCT02430103. [Accessed September 27, 2015].
- Riley RD, Lambert PC, Abo-Zaid G: Meta-analysis of individual participant data: rationale, conduct, and reporting BMJ 2010;340:c221.
- Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L, Stemmer SM. Dose-dense chemotherapy in nonmetastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 2010;102:1845–54.