Abstract
Based on the results from the DBCG 82 trial, breast conserving therapy (BCT) has been implemented as standard in Denmark since 1989, and today constitutes more than 70% of the primary treatment. Our aim was to evaluate the implementation of BCT as a routine procedure in patients treated according to the DBCG 89 program and compare recurrence pattern and survival both overall and when separated in age groups, with the results from the randomized DBCG 82 TM trial. Material and methods: A total of 1847 patients treated between 1989 and 1999 were included in a retrospective population-based cohort study. Data from the DBCG database were completed via search through the Danish Pathology Data Bank and medical records. Results: Median follow-up time was 17 years. At 20 years the cumulative incidences of local recurrence (LR) and disease-specific mortality (DSM) were 15.3% and 25.8%, respectively. Twenty-year overall survival (OS) and recurrence-free survival were 63.7% and 43.1%, respectively. Subdivided by age groups cumulative incidences at 20 years were LR: 18.9%, 10.5% and 12.4%, and DSM: 28.9%, 18.9% and 28.4% in young (≤45 years), middle-aged (46–55 years) and older (≥56 years) women, respectively. In an adjusted analysis age maintained a significant and independent effect on both LR and DSM. Conclusion: The DBCG 82 TM program was successfully implemented. The women treated with BCT in the DBCG 89 program displayed equal failure pattern and improved survival in comparison with women from the DBCG 82 TM protocol. Occurrence of first failure and mortality varied with age; demonstrated by increased risk of LR, DM and DSM in the young patients and increased risk of DM and DSM in the older patients, compared to the middle-aged patients.
Breast conserving therapy (BCT) was implemented as a standard treatment alongside mastectomy for early stage breast cancer in Denmark in 1989 [Citation1]. Large international trials comparing mastectomy with breast conservation without and with radiation therapy (RT) formed the basis for this decision [Citation2–6]. In Denmark the Danish Breast Cancer Group (DBCG) launched the DBCG 82 TM trial. This trial randomized early stage breast cancer patients to BCT or mastectomy in the period of 1982 to 1989 [Citation7–9], and the results from the study demonstrated no differences in first failures and survival between the two treatment groups [Citation8,Citation9]. Thus, both nationally and internationally, BCT was found to be a safe procedure and has consequently been implemented into Danish guidelines as a routine procedure since 1989. Today BCT constitutes more than 70% of all local treatments for primary breast cancer in Denmark [Citation10].
Being a less mutilating procedure, BCT enables the patient to keep most of the size and shape of the breast. However, several studies have demonstrated a higher risk of local recurrences (LR) after BCT [Citation11–14]. Factors associated with increased risk of LR after BCT are: positive resection margins, extensive intra-ductal components in the tumor, and young age [Citation12,Citation13,Citation15–18]. At the same time, young patients more often choose BCT over mastectomy [Citation16]. The question that thus remains is: does the increased frequency of LR in young patients reflect an increased risk of dying from breast cancer? So far no final answers have emerged. This might be explained by the relatively small number of young patients included in many studies and the various age cutoffs used to classify patients. The shift from an experimental to a standard treatment can cause selection for certain patients to one treatment rather than to the other, which can then result in different outcomes relative to what was expected based on the original trial [Citation19,Citation20].
The present study aims at evaluating the long-term failure pattern and survival in a Danish cohort treated with BCT in the period of 1989 to 1999, both for the entire cohort and separated by age. The hypothesis is that BCT, as it is implemented in the DBCG 89 guidelines, yields similar results compared to the DBCG 82 trial, in terms of patterns of failure and survival. We have previously reported on late morbidity and cosmetic outcome in patients treated with BCT from the DBCG 89 cohort [Citation21].
Material and Methods
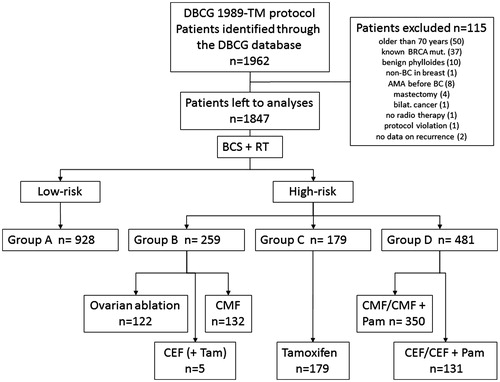
Patients treated with BCT according to the DBCG 89 guidelines from 1989 to 1999 were chosen for at cohort study. Compared to an “ordinary” breast cancer population we enriched the study cohort with patients ≤45 years by including all patients in this age group from the whole country. Patients >45 years were only included from two Danish regions. In this way 1962 early stage breast cancer patients, evenly distributed below and above the age of 45 years, were identified through the DBCG database. As 115 women were not fulfilling the original inclusion criteria in the DBCG 89 guidelines they were omitted, leaving 1847 patients to be analyzed further (). The inclusion and exclusion criteria are provided in the Supplementary material (available online at http://dx.doi.org/10.3109/0284186X.2016.1156741).
DBCG 89 guidelines
The DBCG 89 guidelines have been described in details previously [Citation1,Citation22–26]. Danish women with early stage breast cancer were allocated into low- and high-risk groups independent of the surgical procedure (mastectomy or breast conservation) and further into treatment groups (). Low-risk patients had no lymph node involvement (N0), a tumor size ≤5 cm (from 1998 ≤2 cm), and malignancy grade I tumors [if invasive ductal carcinoma (IDC) and pre-menopausal status], otherwise any grade. These patients entered group A. High-risk patients had positive lymph node involvement (N+) and/or a tumor size >5 cm (from 1998 >2 cm) and were allocated to the following treatment groups: group B, if IDC malignancy grade II–III (irrespective of tumor size), or if pre-menopausal and ER positive, group D, if pre-menopausal and ER negative or unknown, group C, if post-menopausal and ER positive; or group D, if post-menopausal and ER negative.
Figure 1. Patients included, excluded, and allocated into risk groups and treatment. Patients to be included in the cohort, treated according to the DBCG 89 program and identified through the DBCG database (n = 1962). Patients excluded from the cohort (n = 115). Patients left for analyses (n = 1847) separated into low- and high-risk groups and further into treatment sub-groups.
Treatment
In addition to lumpectomy, all women were treated with axillary dissection with the removal of a minimum of 10 lymph nodes. Whole breast irradiation with 48 Gy in 24 fractions were given to all women [Citation27]. The radiation field was extended to include the axilla (level III) and the infra/supra-clavicular areas, if the disease had spread to these locations (regional RT). In case of narrow or involved margins, a boost dose to the tumor bed of 10–16 Gy in 5–8 fractions were given [Citation27].
Group A received no adjuvant systemic treatment. Group B received CMF or ovarian ablation (OA), group C received Tamoxifen in one or two years, or in six months followed by megastrol in six months. Group D received either chemotherapy alone (CMF or CEF) or in combination with pamidronat. See Supplementary material for details on adjuvant systemic treatment, classification of histological type of tumor, assessment of tumor grade and ER status, and for definitions on the menopausal status.
Follow-up
The DBCG database holds information on the patient from the time of diagnosis and treatment through 10 years of follow-up, unless the patient emigrated, developed a recurrence, died, or wished to exit the follow-up program. The database includes patient, tumor and treatment characteristics along with relevant data on the first recurrence and/or cause of death (see Supplementary material).
In the DBCG database 578 first recurrences were registered within the cohort. Through search in the Danish Pathology Data Bank (PDB) and audit of medical records additional recurrences, distant metastases, and deaths were identified and updated. Two hundred and nineteen first recurrences were found through PDB, hereof 167 later than 10 years from diagnosis, and further 18 first recurrences were found in medical records only (see Supplementary material). All patients were followed until December 1st, 2014 or death. In all, 815 first recurrences were identified in the cohort. Breast cancer was registered as the cause of death if a patient had active disease at the time of death.
Endpoints and statistical methods
The following endpoints were used: isolated local recurrence (LRiso) in the ipsilateral chest wall, residual breast or overlying skin; LR in the area described for LRiso including simultaneous recurrence in any other site (within 30 days); regional recurrence (RRiso) in the ipsilateral axillary and/or the infra-clavicular lymph nodes; loco-regional recurrence (LRR) in the area described for LRiso, RRiso, or in combination; contra-lateral breast cancer (CCiso) in the contra-lateral breast; distant metastases (DMiso) outside the areas defined by LRiso, RRiso and CCiso. Overall survival (OS) was death from any cause, calculated from the date of diagnosis to the date of death. Recurrence-free survival (RFS) was the time until any type of recurrence, another primary cancer, or death from any cause occurred, whichever came first, calculated from the date of diagnosis to the date of the occurrence of any of the failures mentioned. Disease-specific mortality (DSM) was death caused by breast cancer calculated from the date of diagnosis to the date of death. Women with no failures were censored with the last follow-up date registered.
The survival estimates were calculated and graphed using the Kaplan-Meier method. A competing risk model and the cumulative incidence function were used to calculate the cause specific incidences for events with one or more competing events occurring [Citation28]. The Cox regression analysis was used to calculate hazard ratios (HR) for univariate analyses of all the variables relevant for the specific endpoint. Variables with a p-value below 0.15 in the univariate analyses were entered into the multivariate analyses. A cubic spline regression model was used to graph the association between DSM and age groups. The confidence intervals and the standard errors for the relative risks were calculated using the Delta method. Significance levels of 0.05 were two-sided. All statistical analyses were performed in Stata version 14.0 (Stata Corp LP, College Station, TX, USA).
The Danish Health and Medicine Authority (Journal no 3-3013-341/1) and the Danish Data Protection Agency (Case # 2010-41-4310) approved this study.
Results
The median observation time was 17 years, range 5 months–25 years. The median age of the cohort was 46 years (range 22–70 years). At the time of diagnosis 1145 women (62%) were pre-menopausal and 1228 women (67%) were node negative. The median tumor size was 15 mm (1–70 mm). IDCs made up 86% of all tumors, and ER positive tumors comprised 57%. shows the patient, tumor and treatment characteristics.
Table 1. Patient, tumor and treatment characteristics in the cohort and in age groups.
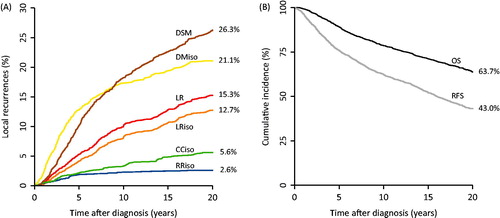
At the time of evaluation, 1206 women (65%) were still alive and 474/641 deaths were caused by breast cancer. The first failures of interest are shown in . In all, 277 LR had developed; 233 as LRiso. DMiso developed in 387 patients as first failures. The cumulative incidences at 10 and 20 years for chosen failures were: LR 9.9% and 15.3%, DMiso 17.3% and 21.1%, and DSM 18.0% and 25.8%, respectively (). Survival at 10 and 20 years were: OS 78.6% and 63.7%, and RFS 62.4% and 43.1%, respectively (). The comparable numbers from the DBCG 82 TM cohort were: 10-year LR: 8.5%, 10-year DMiso: 18.3%, 20 year OS: 57.8% and, 10-year RFS: 59.5%, thus the results from the present study are equal in terms of pattern of failure and improved in terms of overall and RFS.
Figure 2. Cumulative incidences of first failures and survival endpoints for the entire cohort. A: Cumulative incidences of first failures at 20 years for the entire cohort, calculated in a competing risks model. B: Cumulative overall and recurrence-free survival at 20 years for the entire cohort.
In the multivariate analysis on risk of LR (), pre-menopausal status, tumor size larger than 30 mm, and positive lymph node status significantly increased the risk. CMF and OA significantly decreased the risk of LR. In the multivariate analysis on risk of DSM (), tumor size 11–50 mm, IDC malignancy grade II–III, lobular carcinoma, and a positive lymph node status was found to significantly increased the risk. Treatment with CEF significantly decreased the risk of DSM.
Table 2. Uni- and multivariate analyses of LR and DSM.
DSM was analyzed in women subdivided into age groups of five-year intervals and graphed in a spline curve (). This showed significantly higher HR’s for women ≤45 years and for women >55 years, compared to women aged 46–55 years of age. This substantiated the separation of women into three sub-groups with the following cutoffs: ≤45 years (“younger”), 46–55 years (“middle-aged”), and ≥56 years (“older”).
Figure 3. A: Hazard ratios for disease-specific mortality calculated in 5-year age intervals. B: Cumulative incidence of LR at 20 years in young, middle-aged and older patients. C: Cumulative incidence of distant metastases at 20 years in young, middle-aged and older patients. D: Cumulative overall survival at 20 years in young, middle-aged and older patients. E: Cumulative recurrence-free survival in young, middle-aged and older patients. F: Cumulative disease-specific mortality in young, middle-aged and older patients.
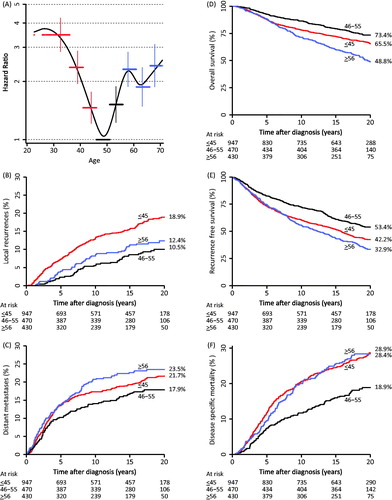
The three age groups were similar for the following characteristics: tumor size, distribution of IDC gr. II–III, number of node negative women, and number of positive lymph nodes. There were significantly more lobular carcinomas among the older women compared to the younger women (p = 0.002). Younger women had more ER negative tumors compared to both the middle-aged and the older women (p = 0.035 and p < 0.001), and less women in the older age group received adjuvant treatment compared to both the younger and the middle-aged women (both p < 0.001) ().
Younger women had a significantly higher risk of LR at 20 years compared to the middle-aged women, 18.9% vs. 10.5%, HR 2.1 ( and ). At 20 years DMiso had developed in 17.9% of the middle-aged women compared to significantly higher frequencies in both the younger and the older women; 21.5% (HR 1.3) and 23.2% (HR 1.5), respectively ( and ).
Table 3. Cumulative incidence proportions and HR of first failures and survival endpoints for the entire cohort and between age groups.
The potential consequences of LRiso were analyzed for subsequent development of distant metastatic disease (). LRiso as a first failure developed in 154/947 (16.3%) younger women compared to 39/470 (8.3%) middle-aged women, relative risk = 2.0 (CI 1.4–2.7). Subsequent development of DM after LRiso occurred in 59/154 younger women (38.3%) compared to 3/39 (7.7%) middle-aged women, relative risk = 4.9 (CI 4.7–5.3). LRiso as a first failure developed in 40/430 (9.3%) older women relative risk = 1.1 (0.46–2.62) compared to the middle-aged women. In addition to this, 13/40 (32.5%) older women subsequently developed a DM after LRiso, resulting in a relative risk = 4.2 (CI 3.6–4.9) compared to the middle-aged women ().
Table 4. Frequencies and proportions of first failures and subsequent metastatic disease.
shows the failure and survival endpoints for the cohort. OS at 20 years in the three age groups (younger, middle-aged and older women) were 65.5% versus 73.4% versus 48.8%, respectively (); significantly worse for the younger (HR 1.4, CI 1.1–1.7) and the older women (HR 2.2, CI 1.7–2.7) compared to the middle-aged women. The cumulative incidences of RFS at 20 years for the younger, middle-aged and older women were: 42.3% versus 53.4% versus 32.9%, respectively (); significantly worse for both the younger (HR 1.4, CI 1.2–1.7) and the older women (HR 1.7, CI 1.4–2.0) compared to the middle-aged women. The DSM at 20 years were 28.9% versus 18.9% versus 28.4% for the younger, middle-aged and older women, respectively (). DSM was significantly worse for the younger (HR 1.4, CI 1.2–1.7) and for the older (HR 1.7, CI 1.4–2.0) women compared to the middle-aged women.
Including ‘age group’ in the univariate and multivariate analyzes showed an independent and significant impact on both LR and DSM when adjusted for tumor size, histological type and grade, ER status, LN-status, and adjuvant treatment (). Younger women had an almost two-times higher risk (HR 2.1, CI 1.5–2.9) and the older women a more than one and a half times higher risk of LR, though non-significant (HR 1.5, CI 1.0–2.2) compared to the middle-aged women, and both younger and older women had significantly increased risks of DSM: HR 1.9 (CI 1.4–2.7) and HR 1.8 (CI 1.3–2.5), respectively, compared to the middle-aged women.
Discussion
Our study confirmed that BCT offered in the DBCG 89 program was at the same level as in the DBCG 82 trial in terms of failure pattern and improved in terms of overall and RFS. The two cohorts were quite similar regarding the distribution of menopausal status and nodal status, but in the DBCG 89 program more patients were considered ‘high-risk’ (49.9% vs. 47.3%), the median age were lower and there was a higher completeness of the registration of all types of events compared to the DBCG 82 TM cohort [Citation9]. The findings in the present study were also superior when compared to other pivotal trials on BCT and mastectomy [Citation6,Citation12,Citation29–32] although these varied from the present study cohort in particular with higher median ages. These trials were conducted in an earlier time period where diagnosis, equipment and treatment modalities were less advanced [Citation33]. Especially better adjuvant systemic treatments may be responsible for the improvements; the use of CEF as an alternative treatment to CMF, optimization of the sequence of chemotherapy and radiation therapy, and longer anti-hormonal treatment [Citation33].
The present study also demonstrated that the patterns of failure vary with age. Similarly Elkhuizen et al. reported on 1360 patients treated with BCT between 1980 and 1994, and reported 10-year risks of loco-regional recurrences of 19% for patients ≤45 years compared to 11% for patients aged 46–65 years [Citation34], and showed that LRR had a major impact on both distant metastases and OS with a relative risk >4 compared to patients without LRR.
The 20-year follow-up report from the Boost Trial (EORTC 22881-10882) [Citation11] demonstrated a strong correlation between patient age and the risk of a LR; decreasing risk with increasing age. When giving a boost treatment, the risk of a LR was significantly reduced for patients <50 years of age [Citation11]. In the Boost Trial less patients were younger (≤40 years), less were pre-menopausal, and more patients were node negative compared to our cohort. Even so, the fact that the boost treatment reduced LRs significantly in patients younger than 50 years might indicate that younger patients who received no boost dose in our study, would have benefitted from a such irrespective of margin status, as margins in the Boost Trial were all stated to be microscopically negative [Citation11].
An earlier DBCG study [Citation35] on a cohort of 10356 pre-menopausal women diagnosed in the period of 1978 to 1996, with some overlap of patients from our cohort, revealed a significantly increased risk of dying from breast cancer in younger patients (<40 years) compared to patients aged 45–49 years. This increased risk only applied to low-risk patients receiving no chemotherapy. Thus, besides the differences found in the distribution of disease characteristics between the youngest and the oldest of the peri-menopausal women in the study, an independent effect of age was present. The conclusion therefore was that young patients should be regarded as ‘high-risk’ and be treated accordingly, on the basis of age alone [Citation35].
Observations of worse outcome for both younger and old patients compared to middle-aged patients in the present study, has to our knowledge only previously been described in the NSABP-06 trial. In this trial, the “bi-phasic” age pattern had slightly different age cutoffs (≤40 years, 41–60 years, and >60 years) but a similar pattern of poorer survival in both the younger and the older patients [Citation36].
The reason for older patients to have worse disease-specific survival in our cohort seems to be related to the higher proportion of patients who developed distant metastases (23.5% vs. 17.9%), which most likely was explained by fewer of the older patients being treated with adjuvant systemic treatment (33% vs. 43%) compared to the middle-aged patients. In the DBCG 89 guidelines the malignancy grade in IDC was not taken into account for post-menopausal women, implying that a post-menopausal lymph node negative woman with a tumor size below 5 cm received no adjuvant systemic treatment regardless of malignancy grade and ER status. Therefore only 80 (38%) out of 208 older patients with IDC malignancy grade II–III received adjuvant systemic treatment, compared to 60% of the middle-aged patients.
Our findings suggests, that the more readily progressive disease demonstrated in especially the younger women with breast cancer, are easily overlooked, as they are overpowered by a much larger proportion of middle-aged women. We intentionally skewed our cohort to be evenly distributed on each side of 45 years of age, and this should be taken into account, when applying the findings on a normally age distributed population. However, it gave us solid conclusions concerning the effect of age.
Our data on resection margins were incomplete, as was the information on DCIS components in the vicinity of the invasive tumor, and these were therefore not included in the analyzes. The data on ER status may have been slightly biased, in the sense that all patients who at some point developed a distant metastasis at that time, had hormone receptor analyses made, whereas not all patients enrolled in the DBCG 89 program were examined with hormone receptor analyses, especially in the beginning of the period.
Patients with known BRCA mutations were omitted from inclusion in our study. This decision was based on the fact that the prevalence of hereditary breast cancer is increasing with younger age [Citation37,Citation38], and based on our preference for the analyses on age differences to be cleared from influence by hereditary factors as far as possible.
A paradoxical drawback for long-term studies is that the results are primarily of historical interest. At the time of diagnosis and treatment in the DBCG 89 program, approximately 50% of the patients with early stage breast cancer received adjuvant systemic treatment, compared to more than 90% of the patients today [Citation10]. In a review from 2009 among others based on data from an EBCTCG meta-analysis [Citation39,Citation40] it was emphasized that the treatment with endocrine therapy reduced the five-year risk of LR with 50%. The authors also highlighted that treatment with poly-chemotherapy reduced LRs by more than 30%, and that this association was age-dependent with younger patients yielding the most benefit from chemotherapy. Based on these effects it seems reasonable to expect that most of the LRs seen in earlier cohorts, such as ours, would be markedly reduced today in line with the much lower incidences of LRs reported in recent studies [Citation18,Citation41,Citation42]. Also the differences between ages in particular may be less obvious today, since the effect of adjuvant treatment has been shown to have the greatest effects in younger patients [Citation40].
Studies searching for factors that can explain the age-dependent effect on outcome are needed – in particular studies focusing on breast cancer intrinsic subtyping seem promising. A study by Anders et al. found a significant association between intrinsic subtypes and age with a higher proportion of younger women (≤45 years) having Basal-like and Her2-enriched subtypes compared to older women [Citation43]. For a large proportion of the patients in our study, we have obtained paraffin-embedded tissue blocks from both the primary tumor and from the LR (if one had occurred). These blocks are currently being analyzed with the aim of categorizing tumors into intrinsic subtypes.
Generally, the view upon retrospective cohort studies has not been as well reputed as the view upon randomized clinical trials (RCT). RCTs precludes possible biases and confounders by the randomization process itself and are therefore considered to produce the highest level of evidence [Citation19,Citation44]. Yet, due to the strict criteria of eligibility, RCTs often enroll younger patients and patients with less comorbidities among others, thereby reducing the general applicability and minimizing the external validity [Citation19]. As a worthy alternative, cohort studies can provide data from larger number of patients with longer observation time and with a wider generalizability. The latter mainly possible due to less rigid eligibility criteria, proper study designs, and when the data collected are of high quality [Citation19,Citation20]. We believe that our study features these qualities. The trials run by the DBCG are nationally very homogenous with a high degree of adherence from all specialties and departments and as a result of this, very high quality of data is provided. In the DBCG, data from the clinical trials are processed and converted into national guidelines as soon as the trial results are available. And owed to the fact that the guidelines have the same high degree of adherence, nationally, high quality data on par with the data originating from clinical trials are provided from the DBCG programs outside the trials [Citation10,Citation45].
In conclusion, we found that the implementation of BCT as a standard treatment in the DBCG-89 TM national guidelines was successful with equal patterns of failure and improved survival compared to the DBCG 82 TM trial. In addition we demonstrated that the risks of local and distant failure, as well as the risk of death, were at much lower levels in the middle-aged women than in the younger and the older women. These results emphasizes that both the younger – and the older women treated with BCT, must receive the adequate tailored treatments (boost, adjuvant systemic treatment), that fits the characteristics of their age group.
Funding information
Financial support was supplied by the Danish Cancer Society, The Danish Center for Interventional Research in Radiation Oncology (CIRRO), and Aarhus University.
Supplementary_12.2.16.pdf
Download PDF (400.1 KB)Supplementary_12.2.16.docx
Download MS Word (93.6 KB)Disclosure statement
There are no disclaimers
References
- Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, et al. The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008;47:506–24.
- Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665–73.
- Sarrazin D, Le M, Rouesse J, Contesso G, Petit JY, Lacour J, et al. Conservative treatment versus mastectomy in breast cancer tumors with macroscopic diameter of 20 millimeters or less. The experience of the Institut Gustave-Roussy. Cancer 1984;53:1209–13.
- van Dongen JA, Bartelink H, Fentiman IS, Lerut T, Mignolet F, Olthuis G, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr 1992;11:15–18.
- Veronesi U, Saccozzi R, Del VM, Banfi A, Clemente C, De LM, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 1981;305:6–11.
- Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003;98:697–702.
- Blichert-Toft M, Brincker H, Andersen JA, Andersen KW, Axelsson CK, Mouridsen HT, et al. A Danish randomized trial comparing breast-preserving therapy with mastectomy in mammary carcinoma. Preliminary results. Acta Oncol 1988;27:671–7.
- Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr 1992;11:19–25.
- Blichert-Toft M, Nielsen M, During M, Moller S, Rank F, Overgaard M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008;47:672–81.
- Jensen MB, Ejlertsen B, Mouridsen HT, Christiansen P. Improvements in breast cancer survival between 1995 and 2012 in Denmark: the importance of earlier diagnosis and adjuvant treatment. Acta Oncol 2016:1–12. [Epub ahead of print].
- Bartelink H, Maingon P, Poortmans P, Weltens C, Fourquet A, Jager J, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47–56.
- van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143–50.
- Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials.!. Lost Data 2001;19:1688–97.
- Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005;28:289–94.
- Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: Risk factors and impact on distant metastases. Cancer 2006;106:35–41.
- Kroman N, Holtveg H, Wohlfahrt J, Jensen MB, Mouridsen HT, Blichert-Toft M, et al. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer 2004;100:688–93.
- Kurtz JM, Amalric R, Brandone H, Ayme Y, Jacquemier J, Pietra JC, et al. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer 1989;63:1912–17.
- Bodilsen A, Bjerre K, Offersen BV, Vahl P, Ejlertsen B, Overgaard J, et al. The Influence of Repeat Surgery and Residual Disease on Recurrence After Breast-Conserving Surgery: a Danish Breast Cancer Cooperative Group Study. Ann Surg Oncol 2015;22(Suppl 3):476–85.
- Chavez-MacGregor M, Giordano SH. Randomized Clinical Trials and Observational Studies: is There a Battle? J Clin Oncol 2016;34:772–3.
- Henson KE, Jagsi R, Cutter D, McGale P, Taylor C, Darby SC. Inferring the Effects of Cancer Treatment: Divergent Results From Early Breast Cancer Trialists' Collaborative Group Meta-Analyses of Randomized Trials and Observational Data From SEER Registries. J Clin Oncol 2016;34:803–9.
- Lyngholm CD, Christiansen PM, Damsgaard TE, Overgaard J. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol 2013;52:259–69.
- Ewertz M, Kempel MM, During M, Jensen MB, Andersson M, Christiansen P, et al. Breast conserving treatment in Denmark, 1989-1998. A nationwide population-based study of the Danish Breast Cancer Co-operative Group. Acta Oncol 2008;47:682–90.
- Christiansen P, Al-Suliman N, Bjerre K, Moller S. Recurrence pattern and prognosis in low-risk breast cancer patients-data from the DBCG 89-A programme. Acta Oncol 2008;47:691–703.
- Ejlertsen B, Jensen MB, Mouridsen HT, Andersen J, Cold S, Jakobsen E, et al. DBCG trial 89B comparing adjuvant CMF and ovarian ablation: similar outcome for eligible but non-enrolled and randomized breast cancer patients. Acta Oncol 2008;47:709–17.
- Andersen J. Tamoxifen for one year versus two years versus 6 months of Tamoxifen and 6 months of megestrol acetate: a randomized comparison in postmenopausal patients with high-risk breast cancer (DBCG 89C). 2008.
- Nielsen KV, Ejlertsen B, Moller S, Jorgensen JT, Knoop A, Knudsen H, et al. The value of TOP2A gene copy number variation as a biomarker in breast cancer: Update of DBCG trial 89D. Acta Oncol 2008;47:725–34.
- Overgaard M, Christensen JJ. Postoperative radiotherapy in DBCG during 30 years. Techniques, indications and clinical radiobiological experience. Acta Oncol 2008;47:639–53.
- Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudovalues of the cumulative incidence function. Biometrics 2005;61:223–9.
- Arriagada R, Le MG, Guinebretiere JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol 2003;14:1617–22.
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–41.
- Jacobson JA, Danforth DN, Cowan KH, D'angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995;332:907–11.
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–32.
- Mouridsen HT, Bjerre KD, Christiansen P, Jensen MB, Moller S. Improvement of prognosis in breast cancer in Denmark 1977-2006, based on the nationwide reporting to the DBCG Registry. Acta Oncol 2008;47:525–36.
- Elkhuizen PH, van de Vijver MJ, Hermans J, Zonderland HM, van de Velde CJ, Leer JW. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys 1998;40:859–67.
- Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study BMJ 2000;320:474–8.
- Fisher ER, Anderson S, Tan-Chiu E, Fisher B, Eaton L, Wolmark N. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer 2001;91:1679–87.
- Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999;91:943–9.
- Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer 2000;83:1301–8.
- Clarke M, Collins R, Darby S, Davies C, Evans V, Godwin J, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–717.
- Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiother Oncol 2009;90:14–22.
- Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086–94.
- Poortmans P, Aznar M, Bartelink H. Quality indicators for breast cancer: revisiting historical evidence in the context of technology changes. Semin Radiat Oncol 2012;22:29–39.
- Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 2011;29:e18–20.
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92.
- Jensen AR, Storm HH, Moller S, Overgaard J. Validity and representativity in the Danish Breast Cancer Cooperative Group-a study on protocol allocation and data validity from one county to a multi-centre database. Acta Oncol 2003;42:179–85.
