To the Editor,
Medullary thyroid cancer (MTC) is a rare neuroendocrine cancer arising from the parafollicular C cells of the thyroid [Citation1]. For patients with metastatic disease, standard treatment modalities include local treatments (radiofrequency ablation, radiation therapy, embolization) and systemic treatments [chemotherapy and more recently tyrosine kinase inhibitors (TKIs) targeting the RET protein [Citation2,Citation3].
Conventional chemotherapies may still be indicated for TKI non-responders, patients with rapidly progressive tumors or those presenting contraindications for these new drugs. A consensus has not yet been reached for a chemotherapeutic regimen in advanced MTC, as experience has been limited to case reports or case series, with response rates (RR) ranged from 0% to 25% and for periods of up to a few months [Citation4].
Here, we report a series of four cases with rapidly progressive metastatic MTC treated by a combination of an antimetabolite agent (5-FU) and an alkylating agent (dacarbazine). Unexpected prolonged responses with significant symptomatic improvements occurred in two patients.
Material and methods
Patients and chemotherapy
Four patients with rapidly progressive advanced MTC were treated with 5-FU and dacarbazine between 2011 and 2014. Chemotherapy was administered every two weeks, except for one patient (every three weeks). The 5-FU dose was 400 mg/m2 + 2400 (bolus injection then 2400 mg/m2 infused over two days) and the dacarbazine dose was 400 mg/m2 (perfused in two hours). Calcium levofolinate (200 mg/m2) was administrated before 5-FU. The study was conducted according to Helsinki guidelines and was approved by the ethical committee of our institution.
Efficacy assessment
Serum calcitonin (Ct) was measured by an immunoradiometric method (IRMA; Cisbio International) and serum carcinoembyronic antigen (CEA) was measured by a method of immuno-chemiluminescence (LOCI, Dimension Vista, Siemens).
We analyzed the tumor responses obtained in accordance with Response Evaluation Criteria In Solid Tumors (RECIST) v1.1.
RET mutation analysis
Tumor DNA was isolated using the EPICENTER Tissue DNA extraction kit (Tebu Bio). RET gene mutations were analyzed by PCR and sequencing.
O6-methylguanine-DNA methyltransferase Status
As previously described [Citation5], O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation status was studied using methyl-specific PCR and pyrosequencing, performed using a PyroMark Q96 MGMT kit (Qiagen, Courtaboeuf, France) on a PSQTM96 MA system (Biotage, Uppsala, Sweden).
Results
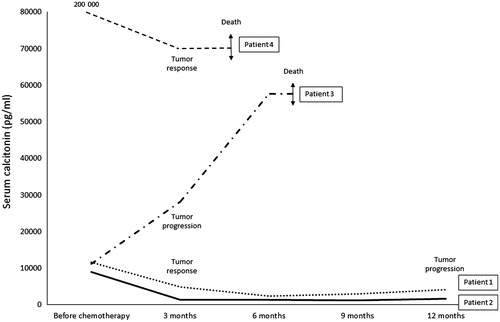
Before treatment, all patients had a rapidly progressive metastatic disease (time of tumor evolution inferior or equal to three months and Ct doubling-time inferior or equal to three months). The median duration of treatment was 10 (5–24) months. No major side effects (grade 3 or 4) were reported. The four patients’ baseline characteristics/clinical, biological and morphological responses are described in Supplementary Tables I and II (available online at http://www.informahealthcare.com). As a whole, the RR was 75% with three partial responses of −50%, −55% and −33%. The median PFS was 8.5 months (3–12 months). The median overall survival was 13.5 months (5–27 months). Death was related to cancer in all four cases. Evolution of serum Ct at three months was associated with the morphological response (decrease of Ct for responders; increase for the non-responder) (). RET somatic mutations were found in three responders (M918T for two patients and C634R for one). The MGMT promoter was not methylated in two responders and one non-responder.
Discussion
Compiling the few case series of advanced MTC treated by a combination of dacarbazine and 5-FU previously published (Supplementary Table 3, available online at http://www.informahealthcare.com) [Citation6–10], the global RR was 18% (10 responders of a total of 55 patients, however RRs were highly variable between series). WHO criteria were used to evaluate tumor response (instead of RECIST in our series).
In these previously published studies, 5-FU was administered as a bolus for three or five consecutive days, whereas in our series 5-FU was administered as a bolus followed by a two-day continuous infusion with more frequent courses (every 2–3 weeks).
The patients selected in this series had a very rapidly progressive disease and there have been reports of better responses to chemotherapy in patients with highly proliferative tumors in digestive neuroendocrine tumors [Citation11].
In neuroendocrine tumors, MGMT status has been associated with responses to alkylant-based chemotherapy [Citation5]. To date, MGMT status has not been studied in MTC. However, the MGMT promoter was not methylated in any of the three tumors tested, including two responders to chemotherapy, suggesting MTC patients can respond well to alkylating agents even without methylation on MGMT.
RET somatic mutations were found in the three responders, and no mutations were found in the non-responder. The presence of a RET somatic mutation, especially M918T, is associated with a worse outcome for MTC patients [Citation12,Citation13], which is consistent with the aggressive forms of MTC in this series.
Thus, long-lasting partial remission can be achieved in some MTC patients using a combination of 5-FU and dacarbazine. Further larger studies are required to establish the roles of both RET somatic mutation and MGMT status in predicting response.
Funding information
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
5FU-dacarbazine_MTC_eng_Supplementary_Table_3.docx
Download MS Word (22.6 KB)5FU-dacarbazine_MTC_eng_Supplementary_Table_2.docx
Download MS Word (19.5 KB)5FU-dacarbazine_MTC_eng_Supplementary_Table_1.docx
Download MS Word (19.9 KB)Acknowledgments
We would like to thank Dr Nadia Bouarioua and Dr Dominique Mille for providing us with clinical data and Dr Johan Etievant for his help.
Disclosure Statement
No competing financial interests exist.
References
- Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610.
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134–41.
- Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–46.
- Hadoux J, Pacini F, Tuttle RM, Schlumberger M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol 2016;4:64–71.
- Walter T, van Brakel B, Vercherat C, Hervieu V, Forestier J, Chayvialle JA, et al. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: prognostic relevance and association with response to alkylating agents. Br J Cancer 2015;112:523–31.
- Schlumberger M, Abdelmoumene N, Delisle MJ, Couette JE. Treatment of advanced medullary thyroid cancer with an alternating combination of 5 FU-streptozocin and 5 FU-dacarbazine. Br J Cancer 1995;71:363–5.
- Di Bartolomeo M, Bajetta E, Bochicchio AM, Carnaghi C, Somma L, Mazzaferro V, et al. A phase II trial of dacarbazine, fluorouracil and epirubicin in patients with neuroendocrine tumours. Ann Oncol 1995;6:77–9.
- Nocera M, Baudin E, Pellegriti G, Cailleux AF, Mechelany-Corone C, Schlumberger M. Treatment of advanced medullary thyroid cancer with an alternating combination of doxorubicin-streptozocin and 5 FU-dacarbazine. Br J Cancer 2000;83:715–18.
- Orlandi F, Caraci P, Berruti A, Puligheddu B, Pivano G, Dogliotti L, et al. Chemotherapy with dacarbazine and 5-fluorouracil in advanced medullary thyroid cancer. Ann Oncol 1994;5:763–5.
- Chow SM, Chan JKC, Tiu SC, Choi KL, Tang DLC, Law SCK. Medullary thyroid carcinoma in Hong Kong Chinese patients. Hong Kong Med J 2005;11:251–8.
- Vilar E, Salazar R, Pérez-García J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer 2007;14:221–32.
- Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008;93:682–7.
- Moura MM, Cavaco BM, Pinto AE, Domingues R, Santos JR, Cid MO, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer 2009;100:1777–83.