Abstract
Background: Radiation therapy (RT) is an integral component of the management of gastroesophageal junction (GEJ) tumors. We evaluated the use of implanted radiopaque fiducials as tumor surrogates to allow for more focal delivery of RT to these mobile tumors when using respiratory gating (RG) to reduce motion.
Material and methods: We analyzed four-dimensional computed tomography scans of 20 GEJ patients treated with RG and assessed correlation between tumor and implanted fiducial motion over the whole respiratory cycle and within a clinically realistic gate around end-exhalation. We evaluated fiducial motion concordance in 11 patients with multiple fiducials.
Results: Gating reduced anterior-posterior (AP) and superior-inferior (SI) mean tumor and fiducial motions by over 50%. Fiducials and primary tumor motions were moderately correlated: R2 for AP and SI linear fits to the entire group were 0.54 and 0.68, respectively, but the correlation had strong inter-patient variation. For all patients with multiple fiducials, relative in-gate displacements were below 3 mm; results were similar for eight of 11 patients over the whole cycle.
Conclusion: Implanted fiducial and gross tumor volume (GTV) motions correlate well but the correlation is patient-specific and may be dependent on the location of the fiducials with respect to the GTV.
Radiation therapy (RT) is an integral component of the management of gastroesophageal junction (GEJ) tumors. However, GEJ tumors have been shown to be more mobile with respiration than more proximal esophageal tumors, potentially reducing the accuracy of treatment delivery [Citation1–3]. As patient-specific respiratory-induced tumor excursion is often unknown, the role of motion management techniques for GEJ tumors is unclear, and strategies are still being developed.
Image-guided radiation therapy (IGRT) helps to improve tumor localization and the accuracy of RT delivery. The combination of IGRT with respiratory gating (RG) potentially allows a reduction in the margins around the tumor to account for tumor motion and day-to-day variation in setup, thereby reducing dose to surrounding tissue [Citation4]. As a result of the poor visualization of esophageal and GEJ tumors on standard non-contrast computed tomography (CT) scans or diagnostic kilovolotage films, IGRT depends on the presence of radio-opaque fiducial markers as surrogates for the soft-tissue target for localization and tracking [Citation5]. However, there are limited data on how accurately fiducial markers represent GEJ tumor motion and whether fiducial-guided IGRT can improve RT for these patients.
Our goal was to use the images from four-dimensional computerized tomography (4DCT) to measure the respiratory motion of GEJ tumors and their implanted fiducials and assess the correlation of these motions.
Methods
Patient characteristics
After obtaining approval from the institutional review board, we retrospectively studied 20 consecutive patients with histologically proven adenocarcinomas of the GEJ treated with RG after fiducial placement between December 2008 and October 2010. Nineteen of these were male. The median age was 55 years (range 43–88 years). Nine tumors were Siewert’s type I tumors (arising in distal esophagus extending to GEJ) and 11 were Siewert’s type II tumors (straddling the GEJ and extending into the gastric cardia). Seven patients had stage II disease, 12 had stage III disease, and one had stage IV disease. The mean tumor volume based on CT was 72 cm3 (range 16–148 cm3).
Fiducials
All patients underwent endoscopic ultrasound (EUS)-guided placement of gold coil (Visicoil™, Bartlett, TN) fiducials prior to simulation (median, 12 days; range 0–55 days). A fiducial marker was backloaded into a 22-gauge needle with the stylet withdrawn approximately 3 cm and the needle tip was sealed with bone wax. Under EUS guidance, the needle was inserted into the tumor, the fiducial was deployed by advancing the stylet, and this was repeated for up to three fiducials. The goal was for the three fiducials to define tumor extent (proximal, central and distal). However, it was not always possible to place three fiducials and often only one or two were implanted. The median number of fiducials was two (range 1–3); four patients had three fiducials, seven had two fiducials, and nine had one fiducial identified at simulation. A total of 35 fiducials were measured and studied in this paper. In almost all cases, the fiducials were inside or just on the boundary of the gross tumor volume (GTV). In one patient, with three fiducials, one was approximately 5 mm superior of the GTV.
Simulation
Patients underwent CT simulation on a General Electric 8-slice Lightspeed system (GE Healthcare, GE Healthcare, Waukesha, WI). Simulation included a contrast-enhanced planning CT scan taken at a voluntary end-exhalation (EE) breath-hold and a registered 4DCT taken with recorded audio coaching customized to match the patient’s breathing pattern. Slice width was 2.5 mm for both studies. The Real-Time Position Management (RPM) system (Varian Medical Systems, Palo Alto, CA) was used to monitor respiratory motion at simulation and treatment.
The GTV comprised a GTVprimary (the region including the primary tumor) and a GTVnodes (involved regional lymph nodes). These GTVs were based on evaluation of pretreatment imaging and endoscopic reports. To generate the clinical target volume, the GTVprimary was expanded by approximately 10 mm radially, 40 mm superiorly along the esophagus to cover para-esophageal and mediastinal nodes, and inferiorly to sufficiently cover the celiac axis lymph nodes, and encompassed the GTVnodes in any of these nodal drainage regions. The heart, lungs, kidneys, stomach, liver, bowel, and spinal cord were contoured following anatomic definitions, as recommended by Matzinger et al. [Citation6].
The 4DCT images were obtained for 10 respiratory phases, which were sorted and reconstructed with vendor software (Advantage 4D, GE Healthcare) aided by an in-house program similar to that described by Rietzel and Chen [Citation7] to correct gross errors in phase assignment relative to the breathing trace. The physician compared the position of the GTV on the planning CT with phase 50% on the 4DCT (EE correlated with phase 50%) to confirm that the simulation CT was obtained at EE and then selected phases around phase 50% to define the gating interval such that the tumor motion was ≤5 mm. This RG window most commonly was set as the interval between the 30% and 70% phases (), but the interval was tightened to 40–60% if necessary, to adequately restrict GTV motion. Extreme tightening of the RG interval was generally avoided because the shorter duty cycle increases delivery time. For this study, the GTVprimary and each of the fiducials were manually delineated by the physician for five phases: 0% [end-inhalation (EI)], 30%, 50%, 70% and 90%. In the following, ‘GTV’ refers only to GTVprimary.
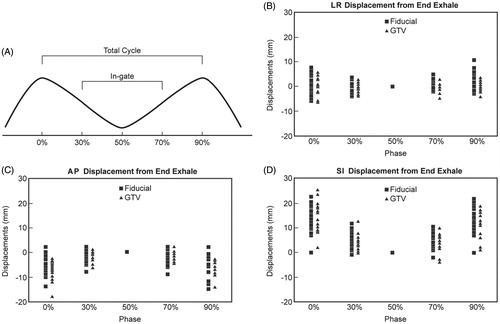
Figure 1. (A) Respiratory cycle definitions, fiducial displacements (square symbols, each phase) and gross tumor volume (GTV) displacements (triangle symbols) from end-exhalation position in (B) left-right (LR), (C) anterior-posterior (Ant-Post), and (D) superior-inferior (Sup-Inf) directions at different breathing phases for all fiducials and GTVs. Each symbol in a phase represents one patient. Positive sign indicates posterior motion in Figure C and inferior motion in Figure D.
Fiducial and GTV motion and correlations
Rigid registration using in-house software was performed to separately register the fiducials and the GTV in each of the phases, 0%, 30%, 70% and 90%, with the corresponding objects in EE (phase 50%). The displacements in the left-right (LR), anterior-posterior (AP), and superior-inferior (SI) directions were recorded as the fiducial and GTV motion from the four breathing phases to EE.
For this analysis, fiducial and GTV displacements between phases 30% and 50% (EE) and between phases 50% (EE) and 70% were measured as representative of “in-gate motion,” while displacements from phases 0% or 90% (EI) to 50% (EE) determined the total respiratory cycle tumor excursion (). Motion within the gate was reported as the larger of the two displacements between phase 30%-to-EE and phase 70%-to-EE. Total cycle motion was reported as the larger of the displacements between phase 0%-to-EE and phase 90%-to-EE.
To validate the use of fiducial motion as a surrogate for GTV motion, the correlation between fiducial and the tumor motion was studied.
Statistical analysis
The relationship between GTV and fiducial motion in the AP and SI directions was modeled with linear regression; displacements in the LR direction were too small for such modeling. For patients with more than one fiducial, the displacements of all the fiducials were averaged. R2 values were calculated to assess: (1) the correlation of GTV and fiducial motion as a pooled analysis of the total patient group; and (2) the correlation of GTV and fiducial motion for each individual patient. To evaluate the concordance of fiducial motion among patients with multiple fiducials placed in different locations in the tumors, the proximal, middle, and distal fiducials displacements in LR, AP, and SI directions among the 11 patients with multiple fiducials placed were measured.
We also evaluated the uncertainty associated with the determination of GTV-fiducial correlation from the simulation 4DCT, which arises from effects of irregular breathing on the phase-sorting method [Citation7,Citation8] and from contouring variability for both GTV and the fiducials. In the absence of these uncertainties, the fiducial-GTV separation should vary smoothly and approximately quadratically with phase: the separation is expected to increase from EI to EE as the diaphragm moves superiorly and tissue decompresses, and to decrease from EE to EI. For 10 of the study patients, we performed a quadratic fit of the fiducial-GTV separation vs phase and estimated the uncertainties as the average over the study population of the root mean square of the fit residuals.
Results
Fiducial and GTV motion
Fiducial and GTV motion ( and ) throughout the total respiratory cycle were greatest in the SI direction with mean displacements of 13.7 ± 5.4 mm and 15.2 ± 5.7mm, respectively. SI motion of the fiducial and GTV within the gating window was reduced to means of 6.0 ± 3.0 mm and 5.6 ± 2.9 mm, respectively. Similarly in the AP direction, fiducial motion was reduced from 6.4 ± 3.7 mm for the total cycle to 3.0 ± 2.2 mm within the gating window, and total cycle GTV motion was reduced from 7.6 ± 3.3 mm to 2.6 ± 1.5 mm. There was a smaller difference, but nonetheless reduced motion, also seen in the LR direction (fiducial, 3.2 ± 2.2 mm to 2.1 ± 1.4 mm gating window; GTV, 3.0 ± 1.7 mm to 1.6 ± 1.2 mm gating window).
Table 1. Total respiratory cycle and in-gate motion for all fiducials and for all GTVs.
Correlation between fiducial and GTV motion
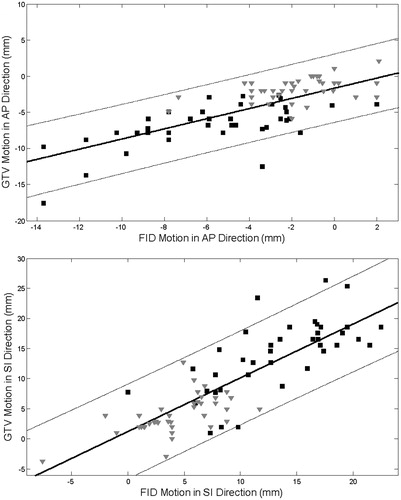
The linear regression results are plotted in . The slopes of the best-fit lines were close to 1.0 (0.70 and 0.89 for the AP and SI fits, respectively). The quality of the fit to the complete data set was only moderate; R2 was 0.54 for the AP and 0.68 for the SI directions. The 95% confidence interval (thin lines) allowed a rather large range in GTV position to be predicted by the fiducial: ±5 mm in the AP and ±10 mm in the SI directions.
Figure 2. Linear regression model of the relationship between the gross tumor volume (GTV) and fiducial displacements in the (AP) direction (A) and SI direction (B). LR displacements were too small for such modeling. Fiducial displacements were averaged for the patients with multiple fiducials. The black squares are total cycle data and the gray triangles are the gated data points. The thin lines indicate the 95% confidence interval.
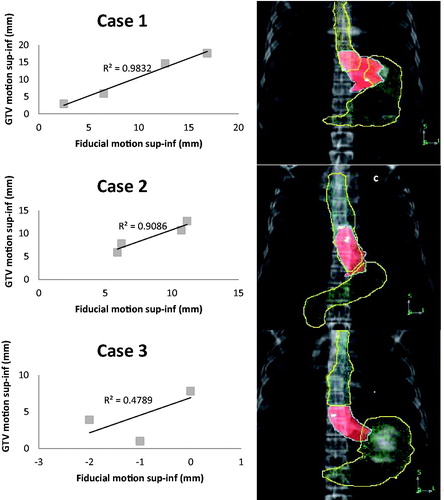
However, the GTV-fiducial correlation had strong intra-patient variation. The median and range of the per-patient AP and SI R2 values were 0.72 (range 0.28–0.97) and 0.92 (range 0.48–0.98), respectively. In Cases 1 and 2, the fiducials measured were placed distally in the GEJ tumor whereas in Case 3, the fiducial was placed proximally in the distal esophagus while the majority of the GTV extended into the GEJ and gastric cardia. Based on the quadratic fit to the fiducial-GTV distance versus phase, the estimated uncertainties (averaged over the 10 patients) in the AP and SI plots were 0.8 mm (range 0.2–1.4 mm) and 1.2 mm (range 0.1–4.0 mm), respectively.
Figure 3. Linear regression of the relation between the gross tumor volume (GTV) and fiducial superior-inferior (sup-inf) displacements for the patient with the highest R2 (Case 1); approximate median R2 (Case 2); the lowest R2 (Case 3). Representative AP digitally reconstructed radiographs (DRRs) showing the placement of fiducial markers (white) with respect to the GTV (central shaded structure), stomach (inferior outlined structure), and esophagus (superior outlined structure) in three representative patients with gastroesophageal junction (GEJ) tumors. Case 1 (a) had excellent GTV-fiducial correlation (R2=0.98), Case 2 (b) had good GTV-fiducial correlation (R2=0.91), and Case 3 (c) had poor GTV-fiducial correlation (R2=0.48). Case 1 is a Siewert’s type 2 GEJ tumor and extends into the gastric cardia where there is the greatest motion; the fiducials are also in the cardia. Case 2 is a Siewert’s type 1 GEJ tumor with the GTV primarily in the distal esophagus approaching the GEJ, the fiducials are also in the distal esophagus. Case 3 is a Siewert’s type 2 GEJ tumor with the GTV extending into the gastric cardia, but the fiducial is higher in the distal esophagus where there is less motion.
Concordance of multiple fiducials
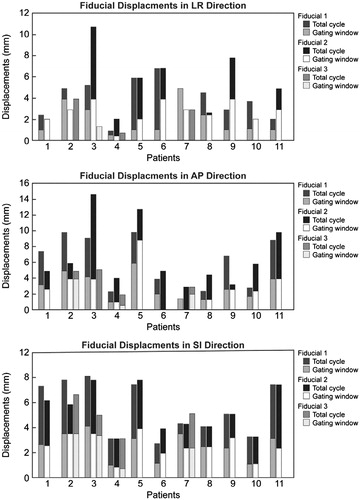
We evaluated the concordance of fiducial motion for the 11 patients with multiple fiducials. If the fiducials rigidly maintained their relative positions throughout the breathing cycle and if there were no other uncertainties, the measured fiducial separation would not change with phase. However, as shown in , the displacements of different fiducials within a tumor were not the same, suggesting that there might be deformation of the tissue in which they were implanted. Within the gating window, discrepancies in the displacements of multiple fiducials were <3 mm in all directions for all the patients and was also ≤3 mm for eight of 11 patients for the total cycle.
Figure 4. Maximum displacements of multiple fiducials in different locations within the tumor of 11 patients in all directions. Bars are a single color if the maximum displacement was the same within the gate as for the total cycle (with one exception: in AP direction, Patient 7, Fiducial 1, the in-gate displacement is 0). In patients with 2 fiducials, Fiducials 1 and 2 are proximal and distal, respectively. In patients with 3 fiducials, Fiducials 1, 2, and 3 are proximal, middle, and distal, respectively.
Discussion
Our study demonstrated that the mean AP and SI tumor and fiducial displacements within the gating window were reduced by more than half compared with the motion over the entire breathing cycle. In addition, we showed that fiducial and GTV motion correlated well, but is patient-specific and may be dependent on the location of the fiducials with respect to the GTV.
A few studies have utilized 4DCT to quantify esophageal motion (see supplement). Zhao et al. evaluated tumor motion in 25 patients with GEJ tumors and found mean peak-to-peak GTV centroid motion of 3.9 ± 2.7 mm LR, 3.8 ± 2.3 mm AP, and 8.7 ± 4.7 mm SI [Citation2]. Yaremko et al. studied 31 patients with distal or GEJ tumors and found that LR motion was 1.2 ± 0.2 mm with no difference in left or right directions, 2.9 ± 0.5 mm for AP with 29 of 31 patients only showing anterior movement relative to EE, and 8.9 ± 0.8 mm for SI displacement, with predominantly inferior tumor movement [Citation1]. Patel et al. studied 30 patients with tumors in the upper (1), middle (4), and distal (25) esophagus. For distal disease, they found that the mean peak-to-peak tumor displacements were 2.5 mm, 3.3 mm, and 8.9 mm in the LR, AP, and SI directions, respectively [Citation3]. More recently, Wang et al. published a study of 29 patients. Of the nine patients with distal disease, they reported peak-to-peak means in LR, AP, and SI directions of 2.4 ± 0.7 mm, 3.0 ± 1.2 mm, and 5.6 ± 1.1 mm, respectively [Citation9]. The above-mentioned studies all measured GTV motion; however, Yamashita et al. quantified motion using implanted fiducial markers instead of GTV. They studied 13 patients with a total of 22 fiducials, and specifically four patients with distal tumors that correlated to seven fiducials in the distal esophagus. They concluded that the margins needed to cover >95% of the primary tumor were 6.8 mm, 6.6 mm, and 13.8 mm in the LR, AP, and SI directions [Citation10]. Lever et al. [Citation11] used sagittal and coronal cine magnetic resonance imaging (MRI) to study esophageal tumor motion in 36 patients. MRI allowed them to image motion over 10 or more breathing cycles. They found that motion depended on the tumor location, with lower esophagus tumors (N = 21) having a significantly larger motion. For these, they reported that a mean (SD) range of 11.5 (3.4) mm in the SI direction and 4.7 (1.7) mm in the AP direction accounted for 95% of the motion and only 2.3 (1.1) mm in the LR direction.
GTV and fiducial motion magnitudes in our study were also largest in the SI direction, which is consistent with all other studies. The total cycle AP and SI motion in our study were considerably larger than the displacements reported in these other studies. This is likely due to the use of recorded audio coaching provided by the RPM system at the time of the 4DCT during simulation, while other studies used quiet, uncoached free breathing. Although our patients were instructed to breath gently, it has previously been found that almost all patients demonstrate larger amplitude with instructed than with uninstructed breathing [Citation12]. The use of audio coaching for gated treatment with RPM requires patient effort and concentration but has been shown to be well tolerated and to improve breathing regularity and reproducibility [Citation12,Citation13]. To maintain consistency with the simulation breathing motion, our patients had audio coaching throughout their daily treatments.
In addition to appropriate prediction of target motion based on the 4DCT at the time of the simulation, accurate localization of mobile GEJ tumors during daily treatment is essential to prevent marginal misses and to limit dose to surrounding normal tissues. EUS-guided implantation of internal fiducials has been shown to be safe and feasible in esophageal cancer, and is becoming more widely used to aid in target localization during radiation treatment [Citation5,Citation14]. The correlation between external marker displacement and movement of tumors in sites affected by respiratory motion has been investigated [Citation15,Citation16]; however, no study has evaluated how well implanted fiducials represent GEJ tumor movement. In this study, we showed that the individual patient’s fiducial and GTV motion had a high correlation, with a median of the AP and SI R2 of 0.72 (range 0.28–0.97) and 0.92 (range 0.48–0.98), respectively. Interestingly, the range in the R2 values was large and may have been related to the position of the fiducial in the esophagus or GEJ with respect to the location of the tumor. Due to limitations in the ability to place the fiducials at the time of the EUS, the fiducials were not always placed in the cardia portion of the tumor. As shown in , the correlation was much better for Cases 1 and 2, where the fiducials were placed more distally and within the GTV. However, for Case 3, the fiducial was placed in the distal esophagus where the motion was much less than the motion of the GTV, which extended into the gastric cardia, leading to an R2 of only 0.48. IGRT accuracy can therefore be improved by working closely with the gastroenterologists who place the fiducials to inform them of optimal placement with respect to the tumor location and not using RG when fiducials are not suitably placed. Placement of multiple fiducials can also help with improving the accuracy of tumor motion assessment and can overcome the potential problem of fiducial migration. We did observe some variability in the distances between fiducials in patients with two or three fiducials between the different breathing phases, suggesting that there was some tumor deformation throughout the respiratory cycle.
Fortunately, the variability was minimal within the gating window in our study (). In general, while internal fiducial markers are helpful surrogates for tumor position, margins based on GTV motion are still needed and, because of deformation and potential migration, fiducial displacements need to be verified with imaging daily over the course of treatment. At our institution, we perform gated kilovoltage images for daily setup and have more recently implemented intra-fraction imaging to verify fiducial positioning during the delivery of RT.
Despite a longer delivery time, the dosimetric benefits of RG have been well established in the treatment of tumors that are affected by respiratory motion [Citation17,Citation18]. The reduced margins allowed by RG make it possible to safely escalate tumor doses without increasing the risk of normal tissue complications or to better protect normal tissues at conventional doses [Citation19,Citation20]. There were several limitations of this study; most importantly, it was retrospective with a small patient cohort. Moreover, we assessed fiducial and GTV motion only during the initial simulation timepoint and our analysis focused on the planning portions of the treatment process rather than the GTV and fiducial motion over the course of treatment. Fiducial migrations during the treatment course were not assessed, nor were other contributions to gating inaccuracy during treatment [Citation18]. In addition, respiratory motion artifacts due to the acquisition and reconstruction of the 4DCT contributed to the uncertainty of the measurements of the GTV and fiducial displacements. While the quadratic fit calculation estimated that the error attributable to distortions in the fiducial and GTV positions due to phase binning of the 4DCT was small, we could not entirely eliminate these uncertainties in our calculations of the fiducial-GTV correlations. Patients should be strongly encouraged to breathe regularly during the 4DCT to minimize artifacts. In some cases, the physician might choose to contour the GTV at EE and the entrance and exit phases of the gate to evaluate the correlation for an individual patient. In the future, respiratory-imaging artifacts may be reduced by algorithms, such as that described by Hertanto et al. [Citation13], and accurate deformable contour propagation would reduce the labor of additional contouring. Moreover, with the use of cine MRI, we may be able to better characterize the motion of the esophagus [Citation11].
In conclusion, respiratory motion of GEJ tumors is substantial with mean SI GTV and fiducial excursions of 15.2 ± 5.7 mm (maximum 25.4 mm) and 13.7 ± 5.3 mm (maximum 22.5 mm) respectively. Motion in all directions, especially SI, was reduced within the gating window, which supports the use of RG for GEJ tumors. Linear correlation between GTV and fiducial motion was moderately good for the entire study cohort and excellent for some individual patients. This indicated that reduced margins together with implanted fiducials for IGRT positioning of gated GEJ patients is reasonable. As the correlation between the fiducials and the GTV was patient-specific, each patient’s overall fiducial and GTV motion should be assessed on 4DCT to determine if the fiducial can be used as a reliable surrogate. Collaboration with gastroenterologists to plan appropriate placement of fiducials is recommended.
Funding information
This work was supported by the National Cancer Institute Core Grant [P30CA008748].
Suppl.docx
Download MS Word (31.2 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Yaremko BP, Guerrero TM, McAleer MF, Bucci MK, Noyola-Martinez J, Nguyen LT, et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2008;70:145–53.
- Zhao KL, Liao Z, Bucci MK, Komaki R, Cox JD, Yu ZH, et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother Oncol 2007;84:283–9.
- Patel AA, Wolfgang JA, Niemierko A, Hong TS, Yock T, Choi NC. Implications of respiratory motion as measured by four-dimensional computed tomography for radiation treatment planning of esophageal cancer. Int J Radiat Oncol Biol Phys 2009;74:290–6.
- Nelson C, Balter P, Morice RC, Bucci K, Dong L, Tucker S, et al. Evaluation of tumor position and PTV margins using image guidance and respiratory gating. Int J Radiat Oncol Biol Phys 2010;76:1578–85.
- DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, et al. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc 2010;71:1204–10.
- Matzinger O, Gerber E, Bernstein Z, Maingon P, Haustermans K, Bosset JF, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol 2009;92:164–75.
- Rietzel E, Chen GTY. Improving retrospective sorting of 4D computed tomography data. Med Phys 2006;33:377–9.
- Hertanto A, Zhang Q, Hu Y-C, Dzyubak O, Rimner A, Mageras GS. Reduction of irregular breathing artifacts in respiration-correlated CT images using a respiratory motion model. Med Phys 2012;39:3070–9.
- Wang W, Li J, Zhang Y, Li F, Xu M, Fan T, et al. Comparison of patient-specific internal gross tumor volume for radiation treatment of primary esophageal cancer based separately on three-dimensional and four-dimensional computed tomography images. Dis Esophagus 2014;27:348–54.
- Yamashita H, Okuma K, Tada K, Shiraishi K, Takahashi W, Shibata-Mobayashi S, et al. Four-dimensional measurement of the displacement of internal fiducial and skin markers during 320-multislice computed tomography scanning of breast cancer. Int J Radiat Oncol Biol Phys 2012;84:331–5.
- Lever FM, Lips IM, Crijns SPM, Reerink O, van Lier ALHMW, Moerland MA, et al. Quantification of esophageal tumor motion on cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2014;88:419–24.
- Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol 2004;14:65–75.
- Giraud P, Yorke E, Ford EC, Wagman R, Mageras GS, Amols H, et al. Reduction of organ motion in lung tumors with respiratory gating. Lung Cancer 2006;51:41–51.
- Fernandez DC, Hoffe SE, Barthel JS, Vignesh S, Klapman JB, Harris C, et al. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pract Radiat Oncol 2013;3:32–9.
- Gierga DP, Brewer J, Sharp GC, Betke M, Willett CG, Chen GTY. The correlation between internal and external markers for abdominal tumors: implications for respiratory gating. Int J Radiat Oncol Biol Phys 2005;61:1551–8.
- Hoisak JDP, Sixel KE, Tirona R, Cheung PCF, Pignol JP. Correlation of lung tumor motion with external surrogate indicators of respiration. Int J Radiat Oncol Biol Phys 2004;60:1298–306.
- Giraud P, Yorke E, Jiang S, Simon L, Rosenzweig K, Mageras G. Reduction of organ motion effects in IMRT and conformal 3D radiation delivery by using gating and tracking techniques. Cancer Radiother 2006;10:269–82.
- Jang SS, Huh GJ, Park SY, Yang PS, Cho EY. The impact of respiratory gating on lung dosimetry in stereotactic body radiotherapy for lung cancer. Phys Med 2014;30:682–9.
- Ramsey CR, Scaperoth D, Arwood D, Oliver AL. Clinical efficacy of respiratory gated conformal radiation therapy. Med Dosim 1999;24:115–19.
- Wagman R, Yorke E, Ford E, Giraud P, Mageras G, Minsky B, et al. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys 2003;55:659–68.
