To the Editor,
Cisplatin-based chemotherapy has significantly improved treatment outcomes in testicular germ cell cancer (GCC) patients, but is associated with elevated risk of serious long-term complications and preventive measures are an unmet need [Citation1]. Physical exercise is known to mitigate chemotherapy toxicities [Citation2], and current cancer-specific guidelines emphasize the capacity of exercise to ameliorate muscle dysfunction by retaining muscle mass and improving physical function in patients undergoing toxic anti-cancer therapies [Citation3].
Skeletal muscle is regulated through complex molecular mechanisms, and chemotherapy is known to target intracellular pathways involved in muscular protein turnover and function. Particularly, muscle progenitor cells, known as satellite cells, have recently gained attention due to their essential role in repair of damaged muscle [Citation4], as well as a source of new myonuclei for muscle fibers undergoing hypertrophy in response resistance exercise. In the oncology setting, concerns are raised as to whether satellite cells entering the cell cycle in response to resistance exercise would be susceptible to chemotherapy damage potentially resulting in a loss of satellite cells [Citation5], which could have implications for muscle repair and/or the progression of sarcopenia. Moreover, acute intramuscular dysregulation of protein turnover pathways could moderate muscle adaptions to resistance training. With current cancer-specific exercise guidelines supporting the addition of muscle preserving resistance exercise during chemotherapy, it is important to assess whether this combination negatively affects the muscle satellite cell pool [Citation5], and whether chemotherapy is associated with dysregulated pathways involved in myofibrillar protein turnover.
In a randomized controlled trial, our group recently found that GCC patients undergoing standard care lost approximately 2.5 kg of lean mass over nine weeks of cisplatin-based chemotherapy, while resistance exercise tended to attenuate the loss of lean mass and improved muscle strength [Citation6]. Here, we extend on our previous findings with the objective to describe intramuscular adaptations induced by chemotherapy with or without concurrent resistance exercise. Specifically, we present an explorative substudy in subjects who had repeated muscle biopsies taken before and after chemotherapy and/or resistance exercise, in order to evaluate changes in muscle satellite cell content and gene expression in GCC patients and healthy individuals.
Material and methods
The study was a randomized controlled trial (ISRCTN 32132990), described in detail elsewhere [Citation7]. Reports on the overall safety and efficacy of resistance training [Citation6], as well as changes in systemic inflammatory profile [Citation8] from the same study have been previously published.
Subjects
Patients with disseminated GCC belonging to the ‘good prognostic group’ according to international guidelines [Citation9] were eligible. Exclusion criteria were: (1) age <18 or >50 years; (2) evidence of cardiovascular disease (cardiomyopathy, coronary heart disease etc.); (3) chronic disease (diabetes mellitus, chronic obstructive pulmonary disease etc.); and (4) inability to read and understand Danish.
All patients received standard bleomycin-etoposide-cisplatin (BEP) chemotherapy (cisplatin 20 mg/m2 and etoposide 100 mg/m2 daily for five days, and bleomycin (15.000 IE/m2 weekly, administered in a three-week schedule for a total of nine weeks) as well as standard antiemetic treatment with prednisolone (50 mg daily), 5HT3-antagonists and metopimazine during the initial five days of each cycle.
A reference group of age- and body mass index (BMI)-matched healthy male individuals was recruited and screened for the same criteria.
Procedures
Before administration of first dose of chemotherapy, patients had muscle biopsies taken from m. vastus lateralis using the Bergstrom technique with added suction. Samples were immediately mounted with Tissue-Tek, frozen in isopentane cooled with liquid nitrogen, and stored at −80 °C. After baseline assessments, GCC patients were randomly allocated 1:1 to either a resistance training intervention (INT) or usual care control (CON). Subjects in the healthy reference group (REF) had a muscle biopsy taken using same procedure, and were allocated without randomization to resistance training. Post-treatment biopsies were collected from INT- and CON-groups 48 hours after administration of the last dose of chemotherapy. Post-training biopsies in the REF-group were collected 48 hours after the final training session. All post-biopsies were collected ∼2 cm proximal to the pre-biopsy incision site from the same muscle.
INT and REF were allocated to resistance training and performed three weekly supervised sessions for nine weeks comprising 3–4 sets of 10 repetitions at 10–12 RM load in four exercises: leg press, knee extension, chest press and lateral pull down, using stationary equipment (Technogym, Gambettola, Italy).
Muscle biopsy analyses
Study personnel conducting the assessment and analyses of muscle biopsies were blinded to group assignment and time point.
Satellite cells
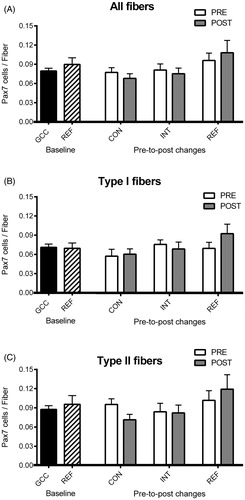
From biopsy samples, serial sections (10 μm) were cut in a cryostat (−20 °C) and stored at −80 °C. Satellite cells were identified on sections stained with antibodies against Pax7, myosin Type I and laminin, as previously described [Citation10]. The number of Pax7 cells associated with all fibers, and Type I and II fibers separately, was determined and expressed relative to the of number fibers included in the assessment.
mRNA analysis
Different mRNA targets were quantified by real time RT-PCR exactly as previously described [Citation11]. Briefly, total RNA was purified from 10 to 20 mg muscle using TriReagent and converted into cDNA using OmniScript Reverse Transcriptase. The cDNA was then quantified with specific primers (Supplementary Table 1, available online at http://www.informahealthcare.com) using Quantitect SYBR Green Master Mix and an MX3005P real time PCR machine. Ct values were related to a standard curve to get copy numbers and RPLP0 was used for normalization.
We were unable to control for the time from the last exercise session to the post-biopsy sampling, and changes in mRNA responses from pre- to post-biopsies are consequently not comparable in the two exercise groups, thus only pre-to-post change in CON is presented.
Statistical analyses
Baseline characteristics were compared using unpaired students t-test and one-way ANOVA for parametric data and χ2-test for categorical data. Satellite cell data is presented as mean ± SEM and changes from pre- and post-biopsies were analyzed by two-way repeated measures ANOVA with group (CON, INT, REF) and time (pre, post) as the two factors. mRNA data were log-transformed before statistical analysis and compared with students t-test, unpaired or paired as appropriate. mRNA data are presented as geometric mean ± back-transformed SEM. All tests were two-tailed and significance level set at 0.05.
Results
At baseline, 30 GCC patients were included and randomized to INT (n = 15) or CON (n = 15), and 19 healthy subjects were allocated to REF (n = 19) (Supplementary Table 2, available online at http://www.informahealthcare.com). For the pre-to-post treatment/training analyses we obtained biopsies for useful analyses in a total of 17 patients (eight from the INT-group and nine from the CON-group) and 13 healthy subjects. For these completers, mean adherence to resistance training was 22.6 sessions (84%) in the INT-group, and 21.2 sessions (78%) in the REF-group.
Satellite cells
At baseline, we found no significant differences in satellite cell number for GCC patients versus healthy controls for all fibers (0.079 ± 0.004 vs. 0.090 ± 0.010, p = 0.299), or for Type I fibers (0.071 ± 0.005 vs. 0.069 ± 0.008, p = 0.867) or Type II fibers (0.087 ± 0.006 vs. 0.095 ± 0.014, p = 0.536, ). Over nine weeks from pre- to post-treatment/training, we found no significant group × time interaction in satellite cell content for all fibers (p = 0.366), Type I fibers (p = 0.300) or Type II fibers (p = 0.132) and no individual effect of either factor was seen ().
Figure 1. Satellite cells. Muscle satellite cell content from muscle biopsies, obtained as baseline (PRE) and after 9 weeks (POST) of chemotherapy and/or resistance exercise. Bars represent mean ± SEM for all fibers (A), and for Type I (B) and Type II (C) fibers at baseline for germ cell cancer patients (black bar, n = 26) and healthy controls (striped bar, n = 15), as well as change from baseline (white bars) to Week 9 (gray bars) for the INT-group (n = 8), CON-group (n = 9), and REF-group (n = 13), respectively. No significant differences were seen (all p > 0.05).
mRNA expression
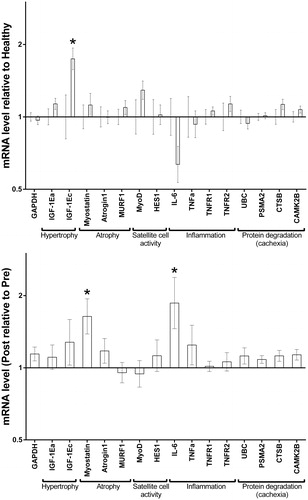
Profiling mRNA markers at baseline in GCC patients and healthy subjects showed no differences, except for increased IGF-1Ec (MGF, . Nine weeks of BEP chemotherapy showed limited chronic effects in the CON-group, except for a slight increase in IL-6 and myostatin, but no changes in TNF-α-related mRNA or other cachexia-related markers were seen (.
Figure 2. mRNA expression. Relative difference in mRNA expression of pathways involved in myofibrillar protein turnover in biopsies obtained at baseline and after 9 weeks of chemotherapy. mRNA data is presented as geometric mean ± back-transformed SEM, at baseline (A) for germ cell cancer patients (n = 26, right bar ± back-transformed SEM) relative to healthy individuals (n = 15, left; set to 1± back-transformed SEM), and change (B) at week 9 (POST, bar ± back-transformed SEM) in fold differences relative to baseline (PRE, set to 1) in the CON-group (n = 9). * = p < 0.05.
Discussion
Attention to cancer patients’ muscular profile has traditionally been confined to the wasting syndrome of cancer cachexia [Citation12], with several reports showing molecular changes associated with tumor-induced subclinical myopathy in muscle biopsies sampled during cancer surgery [Citation13,Citation14]. However, present knowledge of the molecular effects of chemotherapy on skeletal muscle in cancer patients without cachexic features is very limited [Citation15]. Indeed, our study is the first to provide longitudinal biopsy assessments exploring direct effects of chemotherapy on intramuscular profile in cancer patients including the impact of concurrent resistance training.
Satellite cells comprise the resident pool of progenitor muscle cells essential for repair of damaged muscle and serve as a source of new myonuclei. When stimulated by heavy resistance exercise, satellite cells are activated to reenter the cell cycle and proliferate, potentially becoming susceptible to chemotherapy-induced damage [Citation5]. In this context, it is reassuring that we did not find resistance training to result in an acute loss of satellite cells, suggesting that heavy resistance exercise during chemotherapy is safe.
Moreover, the very modest changes in mRNA markers after BEP chemotherapy suggest that potential loss of lean mass and muscle fiber atrophy during treatment is unlikely to be caused by a heavy disturbance of muscle protein turnover regulation, but rather simply an imbalance in protein synthesis/degradation. With no evidence indicating that BEP chemotherapy causes dysregulation of these pathways, the hypotrophy stimulating potential of resistance exercise appears to be unaffected in patients with GCC during chemotherapy.
In conclusion, we found no indication that resistance exercise compromised muscle satellite cell content in GCC patients, and no indication that BEP chemotherapy was associated with significant intra-muscular dysregulation of pathways involved in myofibillar protein turnover. Although these findings are based on a small-scaled, explorative substudy, we believe this information is reassuring for oncologists and therapists involved in the prescription of exercise, including muscle preserving resistance training, to cancer patients during chemotherapy. However, we emphasize that the present data should be interpreted with care due to the explorative nature of the study. Indeed, we believe our findings should be confirmed in larger scaled trials with sufficient power to detect significant differences in these outcomes in order to improve the limited mechanistic understanding of chemotherapy-induced muscle degradation and the capacity of resistance training to ameliorate muscle dysfunction during therapy.
Acknowledgements
This study was supported by the Danish Cancer Society, the Novo Nordic Foundation, the Beckett Foundation, and the University of Copenhagen. The Center of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (DNRF55). The Center for Physical Activity Research (CFAS) is supported by a grant from TRYGFONDEN”. JFC is supported by Research Grants from The Danish Cancer Society and The Capital Region of Denmark. Funding is also gratefully acknowledged from the Nordea Foundation (Healthy Aging grant).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71.
- Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol 2013;52:195–215.
- Buffart LM, Galvao DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev 2014;40:327–40.
- Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011;138:3639–46.
- Clarkson PM, Kaufman SA. Should resistance exercise be recommended during breast cancer treatment? Med Hypotheses 2010;75:192–5.
- Christensen JF, Jones LW, Tolver A, Jorgensen LW, Andersen JL, Adamsen L, et al. Safety and efficacy of resistance training in germ cell cancer patients undergoing chemotherapy: a randomized controlled trial. Br J Cancer 2014;111:8–16.
- Christensen JF, Andersen JL, Adamsen L, Lindegaard B, Mackey AL, Nielsen RH, et al. Progressive resistance training and cancer testis (PROTRACT) -- efficacy of resistance training on muscle function, morphology and inflammatory profile in testicular cancer patients undergoing chemotherapy: design of a randomized controlled trial. BMC Cancer 2011;11:326.
- Christensen JF, Tolver A, Andersen JL, Rorth M, Daugaard G, Hojman P. Resistance training does not protect against increases in plasma cytokine levels among germ cell cancer patients during and after chemotherapy. J Clin Endocrinol Metab 2014;99:2967–76.
- International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997;15:594–603.
- Molsted S, Andersen JL, Harrison AP, Eidemak I, Mackey AL. Fiber type-specific response of skeletal muscle satellite cells to high-intensity resistance training in dialysis patients. Muscle Nerve 2015;52:736–45.
- Agergaard J, Trostrup J, Uth J, Iversen JV, Boesen A, Andersen JL, et al. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? -- a randomized controlled trial. Nutr Metab (Lond) 2015;12:32.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95.
- Zampieri S, Doria A, Adami N, Biral D, Vecchiato M, Savastano S, et al. Subclinical myopathy in patients affected with newly diagnosed colorectal cancer at clinical onset of disease: evidence from skeletal muscle biopsies. Neurol Res 2010;32:20–5.
- Pessina P, Conti V, Pacelli F, Rosa F, Doglietto GB, Brunelli S, et al. Skeletal muscle of gastric cancer patients expresses genes involved in muscle regeneration. Oncol Rep 2010;24:741–5.
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–9.
