The following errors occurred in the article entitled “Delay of cell cycle progression and induction of death of cancer cells on type I collagen fibrils” on pages 167–177 in issue (52) 03 of Connective Tissue Research:
Figure 6. Cell viability of Caco-2 cells on collagen substrates. (A) Fluorescence microscopy of the cells. Cells were cultured on nonfibrous collagen (A-1) or on collagen fibrils (A-2) for 1, 2, 3, and 4 days in media containing 10% serum and then labeled with calcein-AM and propidium iodide (PI). Living cells are stained by calcein-AM (green). Dead cells are labeled with PI (red). (B) Quantification of the cell-viability assay of the Caco-2 cells on collagen substrates. Percentages were determined by counting at least 300 cells per sample in each of three independent experiments. Error bars represent the standard error of the mean.
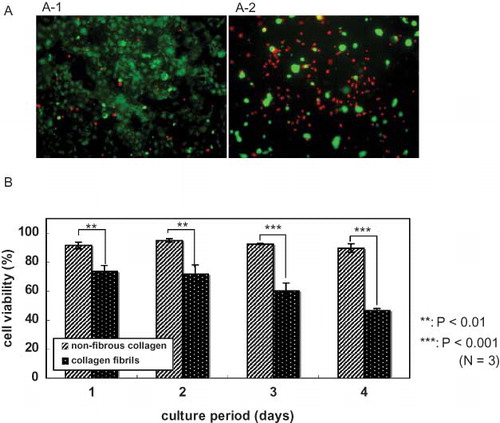
Figure 7. BrdU incorporation of Caco-2 cell on collagen. Cells were cultured on nonfibrous collagen or on fibrous collagen gel for 2 days in media containing 1% serum. After incorporating BrdU for 1 hr, cells were labeled with anti-BrdU antibody (green) and DAPI (blue). (A) Immunofluorescence microscopy of the cells. Caco-2 cells were cultured on nonfibrous collagen (A-1) or on collagen fibrils (A-2). (B) Quantification of BrdU incorporation by Caco-2 cells on collagen substrates. Percentages were determined by counting at least 300 cells per sample.
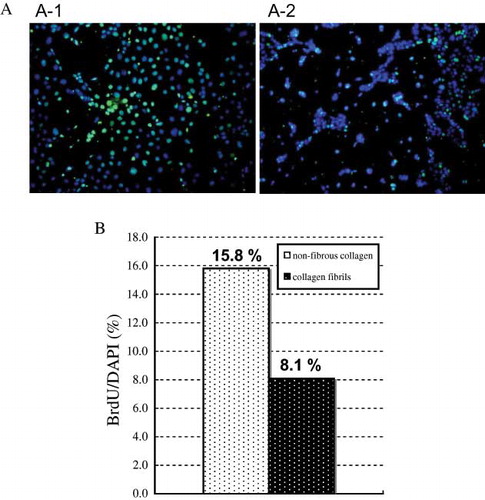
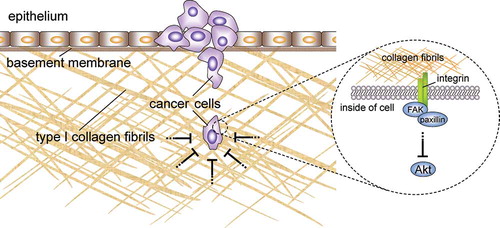
Figure 10. Proposed model for the inhibitory effect of collagen fibrils on cancer cell proliferation. Type I collagen fibrils may function not only as a structural barrier against cancer cell invasion but also as a physiological barrier. The growth and survival of invasive cells that are adhered to collagen fibrils is prevented by the inhibition of the Akt signaling pathway.
The actual title is “Delay of Cell Cycle Progression and Induction Death of Cancer Cells on Type I Collagen Fibrils”.
Also, this article contained figures that were to be set in color but appeared in black and white and contained conversion defects. Below are the three figures along with their appropriate captions:
